The Future of CQV: Trends Shaping the Pharmaceutical Industry Part 2
by Dhika Prameswari, Rida Hadirah Ramli, Nur Syahirah Mustafa
In the ever-evolving landscape of the pharmaceutical industry, the Commissioning, Qualification, and Validation (CQV) processes are increasingly influenced by regulatory evolution and technological advancements. In Part 2 of our exploration, we will discuss key trends shaping the future of CQV, including the transformation of regulatory frameworks, the implementation of integrated quality systems, the importance of real-time monitoring, and the emphasis on continuous improvement. By understanding these developments, industry professionals can better navigate the complexities of CQV, ensuring compliance while enhancing operational efficiency and product quality.

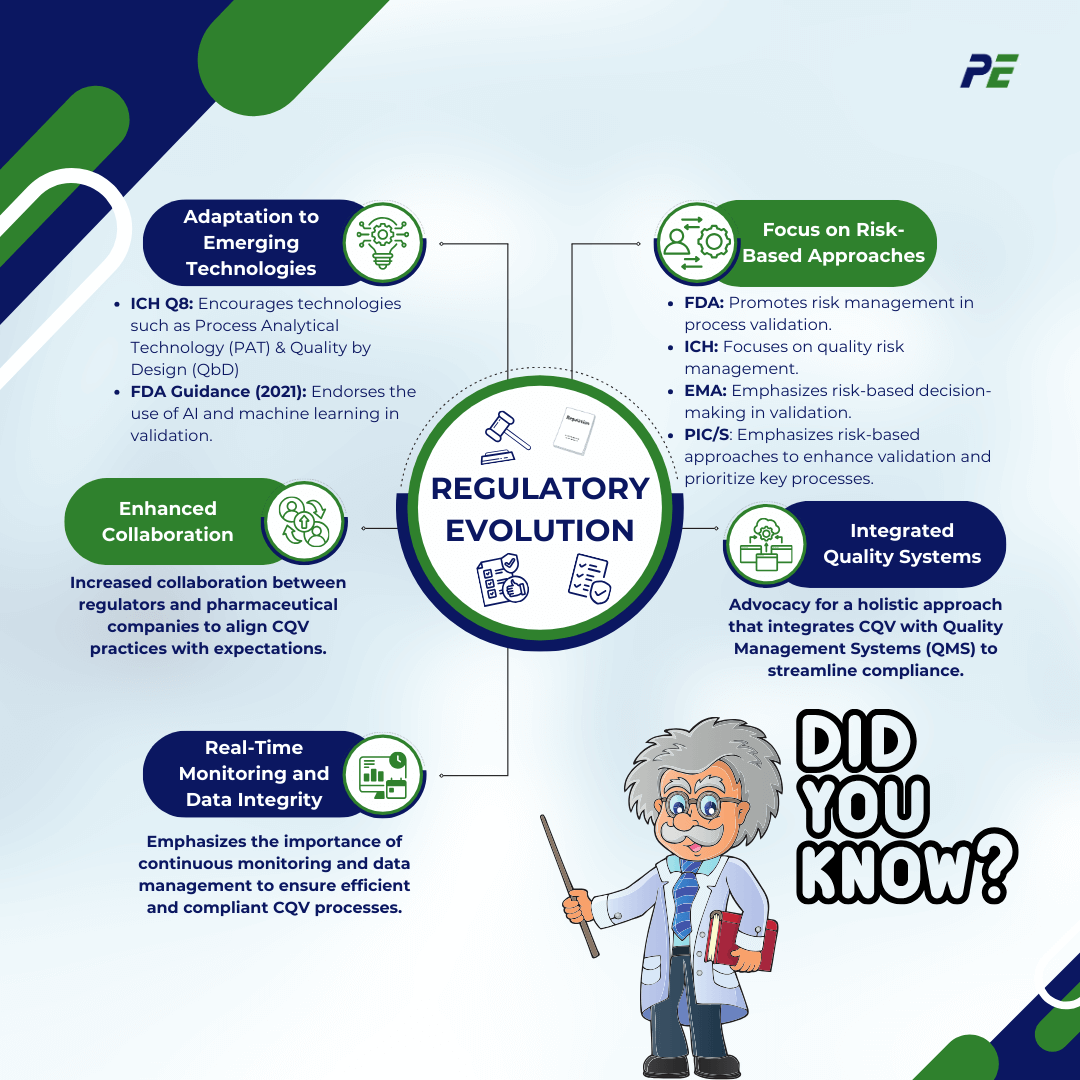
Regulatory Evolution
The pharmaceutical industry is undergoing significant transformations influenced by evolving regulatory frameworks and guidelines. These changes are crucial in shaping the future of CQV processes. Here are some key aspects to consider:
1. Adaptation to Emerging Technologies
Regulatory agencies are recognizing the importance of new technologies like AI, machine learning, and automation in CQV processes. Key guidelines supporting this include:
- ICH Q8 (Pharmaceutical Development): Encourages advanced technologies like Process Analytical Technology (PAT) and Quality by Design (QbD).
- FDA Guidance on AI and Machine Learning (2021): Explores how AI and ML can streamline validation in drug manufacturing.
These guidelines highlight the increasing regulatory support for adopting emerging technologies in CQV processes.
2. Focus on Risk-Based Approaches
Regulatory bodies are shifting towards risk-based frameworks, encouraging companies to prioritize critical aspects of their processes. Below are the regulatory bodies that emphasize risk-based approaches (RBA):
- FDA (U.S. Food and Drug Administration) – The FDA advocates for a risk-based approach in their Process Validation Guidance (2011), which emphasizes understanding product and process variability to manage risks. They also support the use of risk-based principles in their Current Good Manufacturing Practices (cGMPs).
- ICH (International Council for Harmonisation) – The ICH Q9: Quality Risk Management guideline is a global standard promoting risk management throughout the lifecycle of a pharmaceutical product, from development to manufacturing. It focuses on identifying, assessing, and controlling risks, which helps tailor validation efforts.
- EMA (European Medicines Agency) – EMA has adopted risk-based methodologies as part of its guidelines, particularly in the Annex 15: Qualification and Validation document. This encourages the use of risk-based decision-making in validation activities.
- PIC/S (Pharmaceutical Inspection Co-operation Scheme) – The PIC/S Guide to Good Manufacturing Practice (GMP) for Medicinal Products emphasizes the use of risk-based approaches to streamline validation activities and prioritize critical processes.
This approach emphasizes understanding potential risks and allows for tailored validation strategies that optimize resources and improve compliance.
3. Enhanced Collaboration
There is a growing emphasis on collaboration between regulatory agencies and pharmaceutical companies. Early engagement with regulators helps ensure that CQV practices align with regulatory expectations, fostering a more efficient approval process.
4. Integrated Quality Systems
Regulatory guidelines are increasingly advocating for integrated quality management systems that encompass all aspects of production. This holistic approach streamlines CQV activities and enhances overall product quality and compliance.
5. Real-Time Monitoring and Data Integrity
The push for real-time monitoring and data integrity is shaping CQV practices. Regulations are being updated to emphasize the importance of continuous monitoring and data management, ensuring that validation processes remain compliant and efficient.
By understanding these changes in regulatory frameworks and guidelines, industry professionals can adapt their CQV strategies accordingly. This proactive approach not only ensures compliance but also positions organizations to leverage innovations, enhance operational efficiency, and maintain high-quality standards in the pharmaceutical sector.
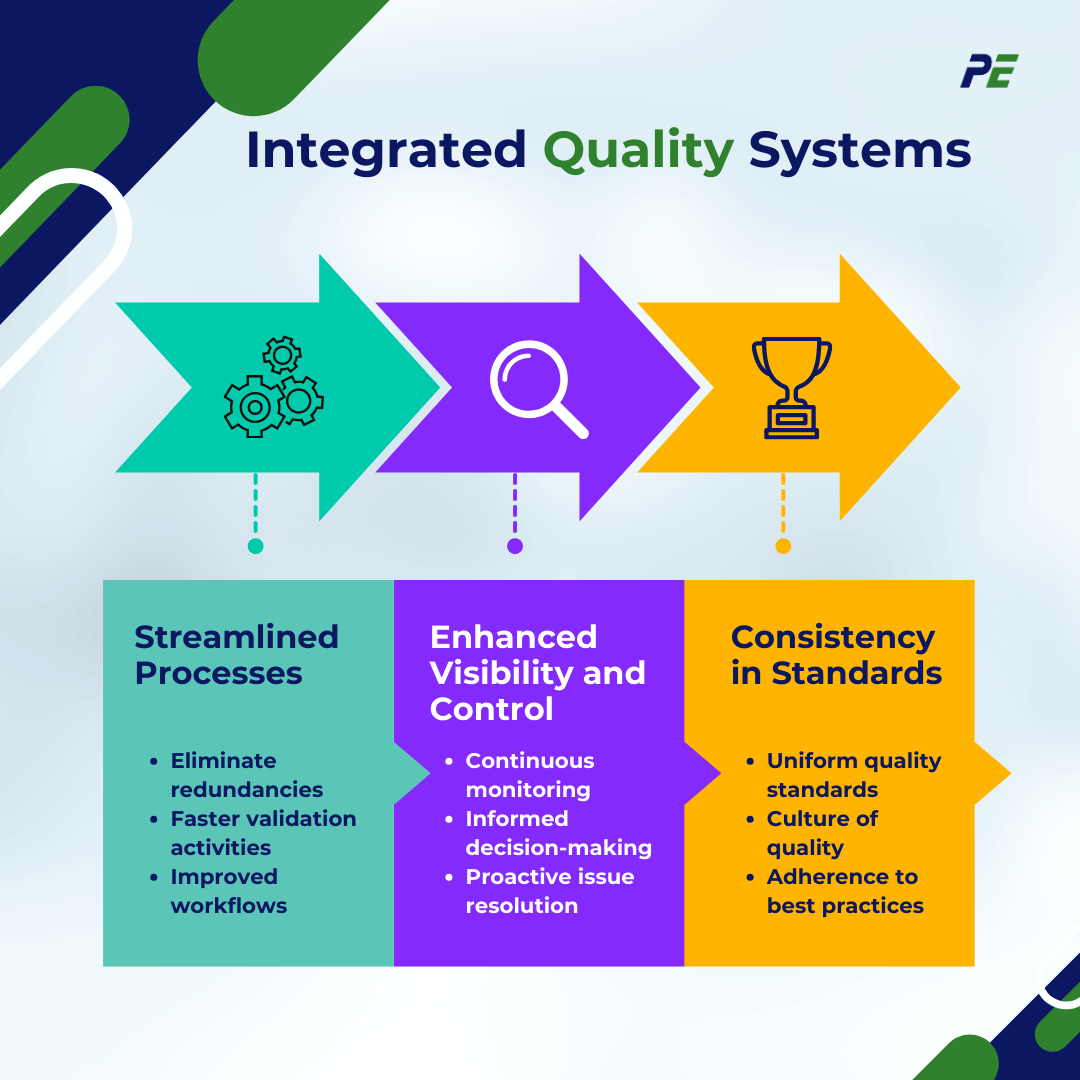
Integrated Quality Systems
The integration of CQV processes with overall Quality Management Systems (QMS) is becoming increasingly important in the pharmaceutical industry. The integration of CQV (Commissioning, Qualification, and Validation) with Quality Management Systems (QMS) aligns with several regulatory guidelines aimed at ensuring compliance, product quality, and operational efficiency. Some key guidelines from regulatory bodies include ICH Q10, US FDA 21 CFR part 820, EU GMP Annex 15, and ISO 13485. Here are several key aspects of this integration:
1. Streamlined Processes
By integrating CQV with QMS, organizations can streamline their processes, reducing redundancies and ensuring that all quality-related activities are aligned. This synergy facilitates a more efficient workflow, allowing for faster execution of validation activities while maintaining compliance with regulatory standards.
2. Enhanced Visibility and Control
A unified approach allows for better visibility into all quality-related data and processes. By consolidating CQV activities within the broader QMS framework, organizations can monitor quality metrics in real-time, leading to more informed decision-making and proactive issue resolution.
3. Consistency in Standards
Integration helps ensure that CQV processes adhere to the same quality standards as other operations within the organization. This consistency fosters a culture of quality across departments, promoting adherence to best practices and regulatory requirements throughout the lifecycle of a product.
The trend towards integrating CQV with overall Quality Management Systems is transforming how pharmaceutical companies approach compliance and quality assurance. By adopting this holistic perspective, organizations can enhance efficiency, improve product quality, and remain agile in an ever-evolving regulatory landscape. This integration ultimately leads to a more robust quality culture, positioning companies for long-term success in the pharmaceutical industry.
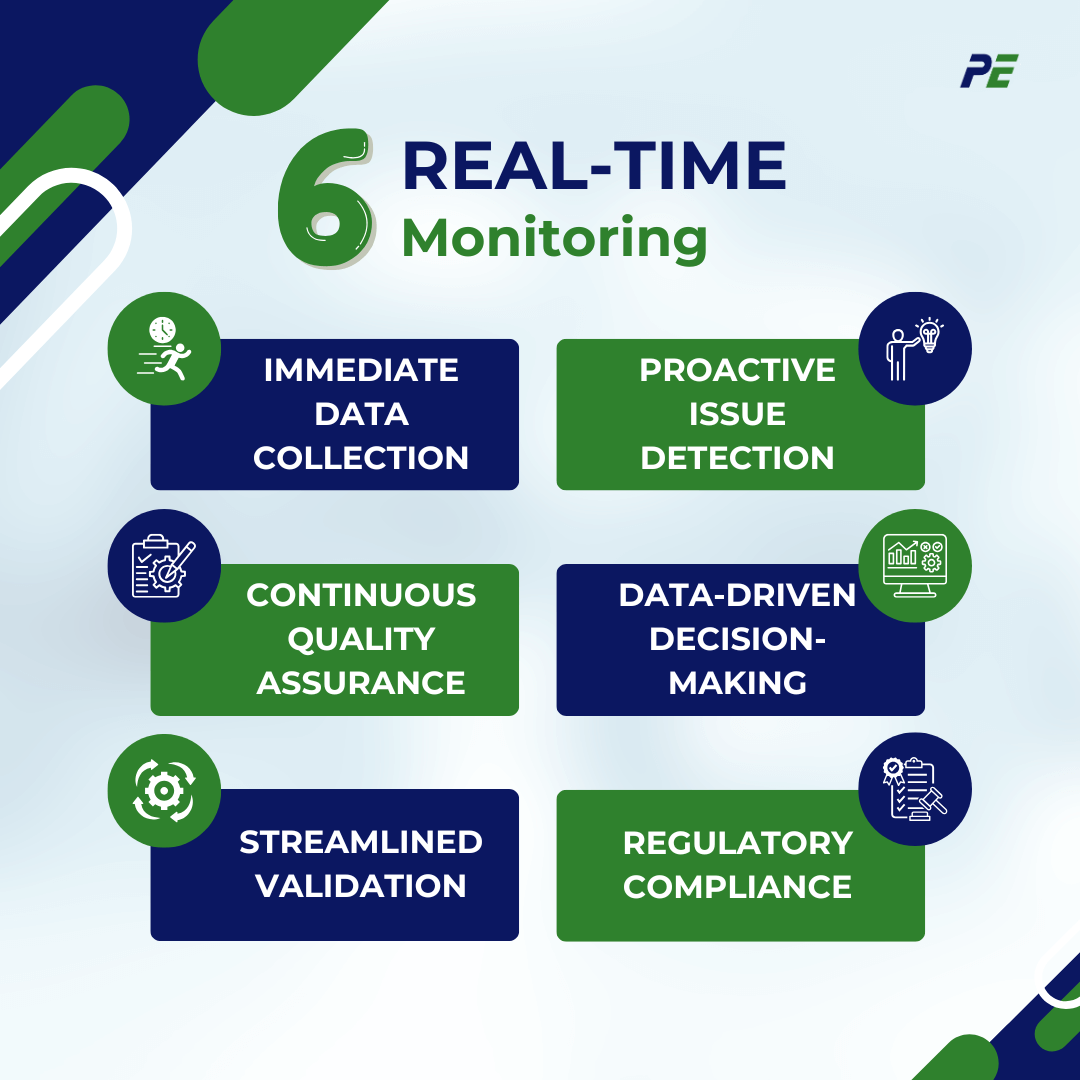
Real-Time Monitoring
The pharmaceutical industry is increasingly adopting real-time monitoring, which significantly enhances proactive quality assurance and validation. This shift offers several advantages:
- Immediate Data Collection: Continuous data gathering from IoT devices and sensors provides instant insights into critical process parameters, enabling quicker decision-making.
- Proactive Issue Detection: Real-time monitoring allows for the immediate identification of deviations from established parameters, allowing teams to respond swiftly to potential quality issues before they escalate.
- Continuous Quality Assurance: This approach supports ongoing oversight of product quality, reducing reliance on periodic checks and facilitating a culture of continuous improvement.
- Data-Driven Decision-Making: Access to real-time data fosters informed decision-making, helping organizations optimize processes and enhance operational efficiency.
- Streamlined Validation: Real-time monitoring simplifies validation processes by reducing manual data reviews, allowing for faster execution of protocols without compromising quality.
- Regulatory Compliance: Comprehensive and auditable data trails from real-time systems make it easier to demonstrate compliance with regulatory standards, simplifying the documentation process. The following regulatory guidelines such as US FDA 21 CFR Part 11 Electric Records; Electronic Signatures and EU GMP Annex 11 (Computerised System).
In summary, the shift towards real-time monitoring is revolutionizing quality assurance and validation in the pharmaceutical industry. By enabling proactive risk management and fostering a culture of continuous improvement, organizations can enhance product quality while ensuring compliance with regulatory expectations.
Continuous Improvement
Continuous improvement initiatives play a crucial role in the evolution of Commissioning, Qualification, and Validation (CQV) practices within the pharmaceutical industry. As regulations and technologies evolve, organizations must adopt a mindset of ongoing enhancement to ensure compliance and optimize processes. Here are several key points highlighting the importance of these initiatives:
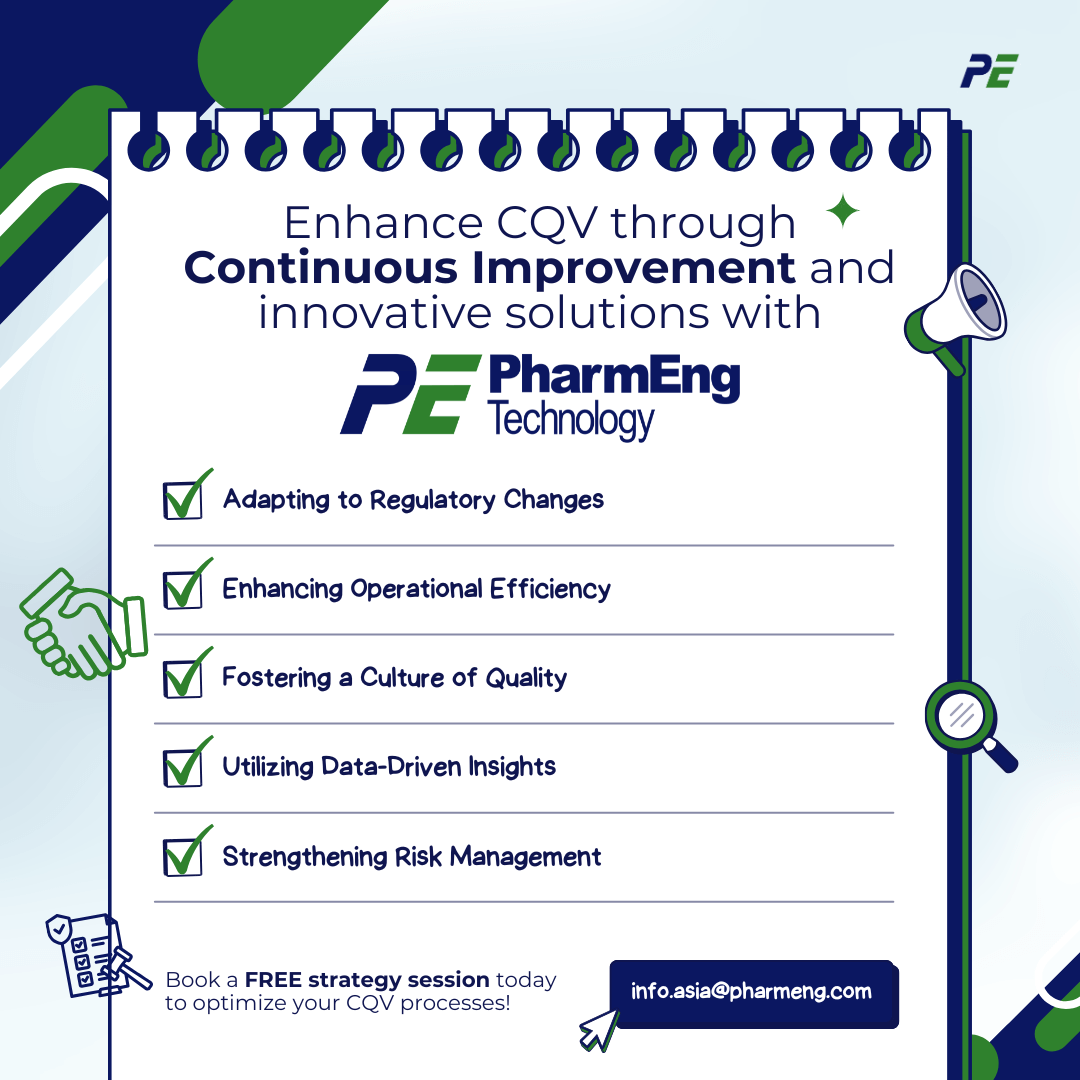
- Adapting to Regulatory Changes: Continuous improvement allows organizations to swiftly adapt to evolving regulatory requirements. By regularly reviewing and updating CQV processes, companies can ensure that they remain compliant and avoid potential regulatory pitfalls.
- Enhancing Operational Efficiency: Initiatives focused on continuous improvement help identify inefficiencies and bottlenecks within CQV practices. Streamlining these processes not only saves time and resources but also enhances overall operational effectiveness.
- Fostering a Culture of Quality: By emphasizing continuous improvement, organizations cultivate a culture that prioritizes quality at every level. This mindset encourages employees to take ownership of quality issues, fostering collaboration and accountability throughout the organization.
- Utilizing Data-Driven Insights: Continuous improvement initiatives often leverage data analytics to identify trends and areas for enhancement. By making data-driven decisions, organizations can implement targeted strategies that lead to measurable improvements in CQV practices.
- Strengthening Risk Management: Regularly assessing and refining CQV processes enhances an organization’s ability to identify and mitigate risks proactively. Continuous improvement enables teams to stay ahead of potential quality issues, ensuring a more robust validation framework.
Continuous improvement initiatives are essential for evolving CQV practices and ensuring ongoing compliance in the pharmaceutical industry. Continuous improvement initiatives are essential for evolving CQV practices and ensuring ongoing compliance in the pharmaceutical industry. The ICH Q10 guideline emphasizes a systematic approach to pharmaceutical quality, encouraging companies to implement continuous improvement throughout the product lifecycle. By fostering a culture of quality, leveraging data-driven insights, and embracing innovation, organizations can enhance their CQV processes, aligning with the ICH Q10 framework. This not only improves product quality but also ensures better regulatory outcomes and long-term compliance in an evolving industry.
Conclusion
In conclusion, the future of Commissioning, Qualification, and Validation (CQV) in the pharmaceutical industry is being shaped by several key trends, including the integration of emerging technologies, the adoption of risk-based approaches, and the alignment with overall Quality Management Systems (QMS). Regulatory bodies are increasingly emphasizing these trends, recognizing their potential to enhance compliance, streamline processes, and improve product quality. Real-time monitoring capabilities and comprehensive data trails are paving the way for proactive quality assurance and simplifying regulatory documentation. As the industry continues to evolve, organizations that embrace these trends will be better equipped to navigate regulatory complexities and achieve operational excellence, ultimately ensuring the delivery of high-quality pharmaceutical products to the market.
Partnering with PharmEng Technology
The future of CQV is rapidly evolving, driven by technological advancements and regulatory changes that are reshaping the pharmaceutical industry. At PharmEng Technology, we are at the forefront of these transformations, helping companies stay ahead of the curve while ensuring compliance and operational excellence. Partner with us to leverage cutting-edge strategies and optimize your CQV processes.
Book your FREE strategy session today by contacting us at info.asia@pharmeng.com or or filling out the form here. Let’s work together to shape the future of pharmaceutical excellence!
About PharmEng Technology
PharmEng Technology is a global consulting firm specializing in pharmaceutical engineering, regulatory affairs, and compliance. With a commitment to quality and innovation, PharmEng Technology provides comprehensive solutions to meet the evolving needs of the pharmaceutical and biotechnology industries.
Contact Information
PharmEng Technology
Email: info.asia@pharmeng.com





