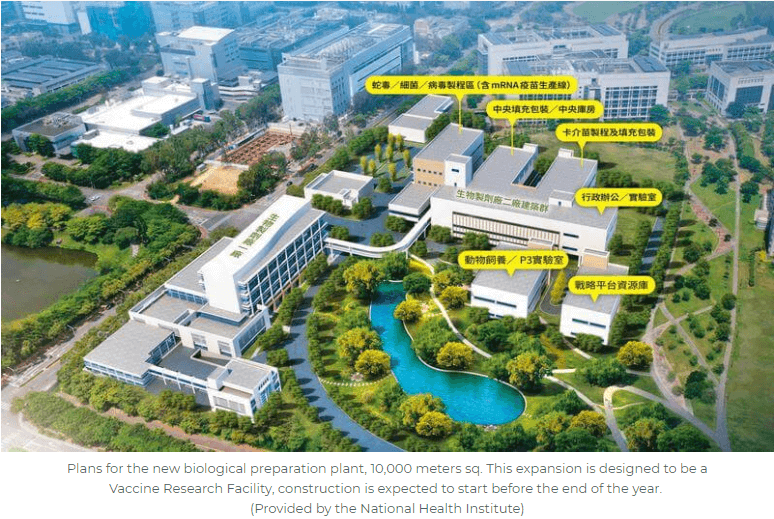
Taiwan’s National Health Research Institute Expansion for Vaccine Facility Begins Construction in 2021
The government of Taiwan and the National Health Research Institute (NHRI) are initiating an expansion project for a Phase 2 facility to establish a vaccine technology platform. The NHRI is Taiwan’s national-level non-profit dedicated to medical research and improved healthcare in Taiwan, partly modeled after the US National Institute of Health (NIH).
PharmEng is proud to announce our participation in a joint venture partnership for the design and validation of VRDC’s second facility. This team is
- Led by: C.C.Hsu Architects & Engineers
- Project Management by: Proceed Engineering Consultants Co., Ltd.
- GMP and Validation by PharmEng Technology

PharmEng Technology had significant involvement with the first stage of the Vaccine Research Centre. In 2005, PharmEng was proud to be a member of a team that built and validated VRDC’s first facility in Zhunan, Taiwan, creating and executing the Master Validation Plan for the facility.
The new facility will cater to existing domestic disease control vaccines, as well as have the capability to develop and manufacture tomorrow’s vaccines.
It will be one of the largest public vaccine facilities in East Asia. PharmEng is honored to aid in strengthening Taiwan’s healthcare system, and the relationships between Taiwan, Canada, the National Health Research Institute, and PharmEng Technology.
An additional thanks to the Canadian Trade Office in Taipei (CTOT) for supporting PharmEng as honorable delegates.





