
Staying Compliant: Navigating Regulatory Changes in CQV Part 2
by Dhika Prameswari, Rida Hadirah Ramli
In the previous article, we explored the regulatory landscape, key regulatory guidelines, and the impact of regulatory changes on CQV processes. Thus, in this second part, we will delve into essential best practices for maintaining compliance and practical strategies organizations can adopt to stay ahead of regulatory updates. Additionally, this article will examine the role of modern technology in enhancing regulatory compliance efforts. By adopting proactive strategies and leveraging advanced tools such as real-time monitoring systems and automated documentation solutions, organizations can streamline validation processes, ensure data integrity, and meet evolving regulatory requirements more efficiently.
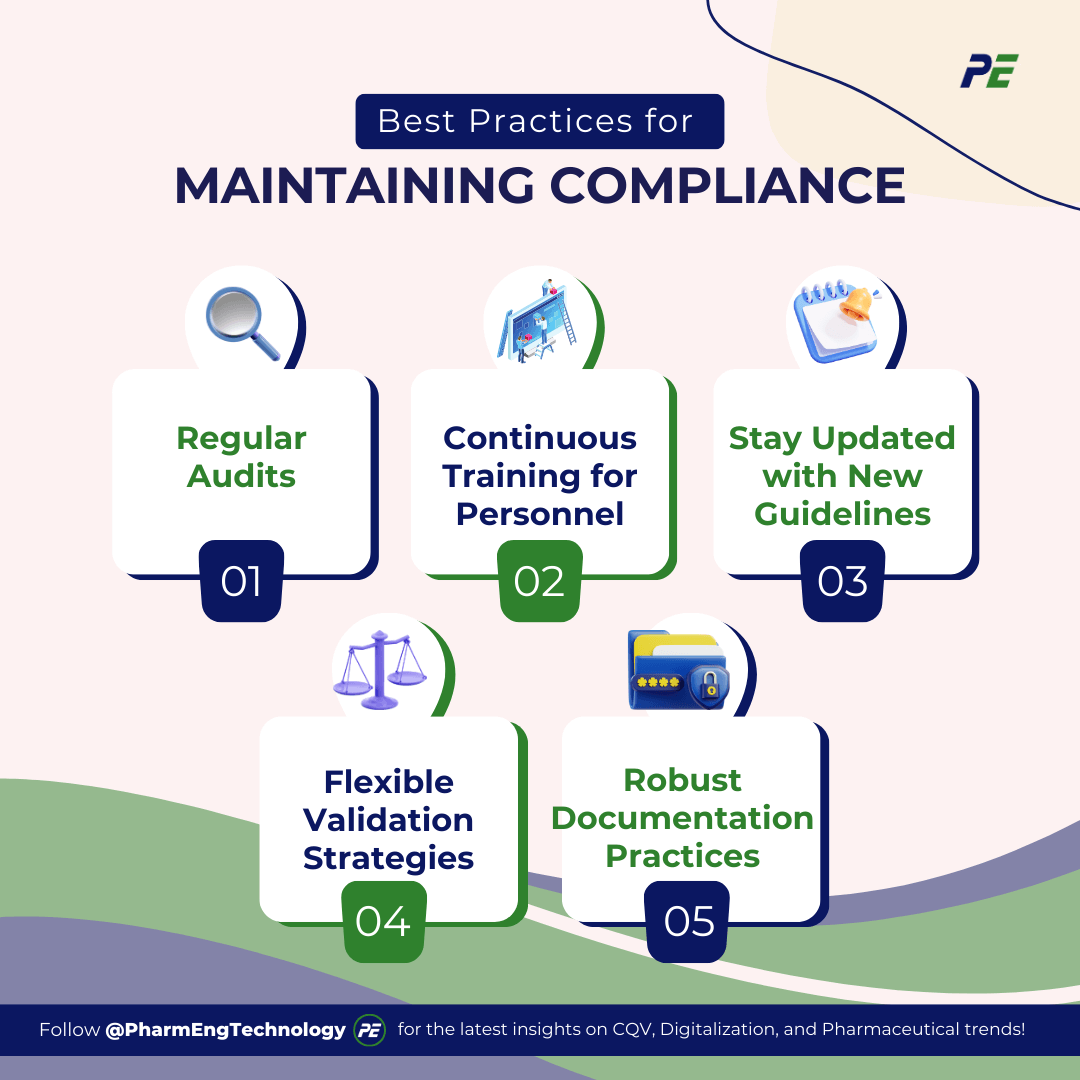
Best Practices for Maintaining Compliance
Maintaining compliance in CQV requires a robust framework built on well-established practices. These best practices form the foundation for ensuring that organizations remain compliant with regulatory changes, safeguard product quality, and uphold patient safety. Here are the core practices every organization should implement:
1. Regular Audits:
- Regular internal audits help identify compliance gaps before they become critical issues and ensure CQV processes align with current regulations.
- Use audit findings to implement corrective and preventive action (CAPA) and continuously improve processes.
2. Continuous Training for Personnel:
- Provide ongoing training for staff on updated regulations and compliance requirements, ensuring they are aware of industry changes.
- Conduct specialized training on data integrity, validation protocols, and any new technology implemented to enhance CQV processes.
3. Stay Updated with New Guidelines:
- Subscribe to regulatory body updates (e.g., FDA, EMA, ICH) to stay informed of new or amended guidelines.
- Implement a system to regularly review and incorporate these updates into CQV procedures.
4. Flexible Validation Strategies:
- Adopt a risk-based validation approach that allows flexibility in adapting to regulatory changes without overhauling entire systems.
- Use continuous verification (CV) and real-time monitoring to ensure processes remain in control and compliant throughout their lifecycle, even as regulations evolve.
5. Robust Documentation Practices:
- Ensure comprehensive, accurate, and timely documentation of all CQV activities as a record of compliance.
- Utilize digital tools to automate documentation management and ensure all changes are traceable, securely stored, and easily retrievable.
By implementing these best practices, organizations can proactively address regulatory changes, and minimize the risk of regulatory violations and inefficiencies in the CQV process.
Practical Strategies and Proactive Actions
While best practices provide a solid foundation, organizations must also be agile in responding to specific regulatory updates and challenges. Here are strategies to ensure a more adaptive and agile compliance framework:
1. Continuous Monitoring of Regulatory Updates
- Proactive Action: Assign a regulatory affairs team or specialist to track updates from regulatory bodies like the FDA, EMA, and ICH.
- Practical Strategy: Subscribe to newsletters, attend industry conferences, and regularly disseminate regulatory changes to all relevant departments. Ensure swift integration of new guidelines into CQV processes.
2. Phased Implementation of Technology
- Proactive Action: Instead of overhauling all systems at once, adopt a phased approach to integrating new technology, such as real-time monitoring and automated solutions.
- Practical Strategy: Spread costs over time and minimize disruption by rolling out new systems gradually. Focus on critical areas first, ensuring they meet regulatory requirements before expanding implementation.
3. Regular Internal Audits with Risk-Based Approaches
- Proactive Action: Conduct frequent internal audits to identify non-compliance risks and implement risk-based approaches to system upgrades and automation.
- Practical Strategy: Prioritize upgrading critical systems that pose the highest compliance risks. Audit findings should be used to drive action plans for remediation before issues escalate.
4. Implement a Strong Data Governance Framework
- Proactive Action: Ensure all CQV data is handled according to ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available).
- Practical Strategy: Adopt automated data capture tools and cloud-based storage solutions that align with regulatory guidelines like 21 CFR Part 11 for electronic records to ensure that all CQV data is securely stored, traceable, and accessible during inspections.
5. Cross-Functional Collaboration
- Proactive Action: Foster collaboration between departments such as Quality, Regulatory, Engineering, and IT to ensure alignment on regulatory expectations.
- Practical Strategy: Organize regular cross-functional meetings to discuss regulatory updates and their impact on CQV. Define collaborative solutions that improve compliance across all departments.
6. Strengthen Documentation Practices
- Proactive Action: Ensure thorough documentation of all CQV processes and activities, with a focus on traceability and transparency.
- Practical Strategy: Use an electronic document management system (EDMS) to maintain centralized, real-time access to CQV documentation, ensuring they are readily available for audits and reviews.
By implementing these proactive actions and practical strategies, organizations can better navigate regulatory changes in CQV, ensuring ongoing compliance, improving operational efficiency, and maintaining high product quality.

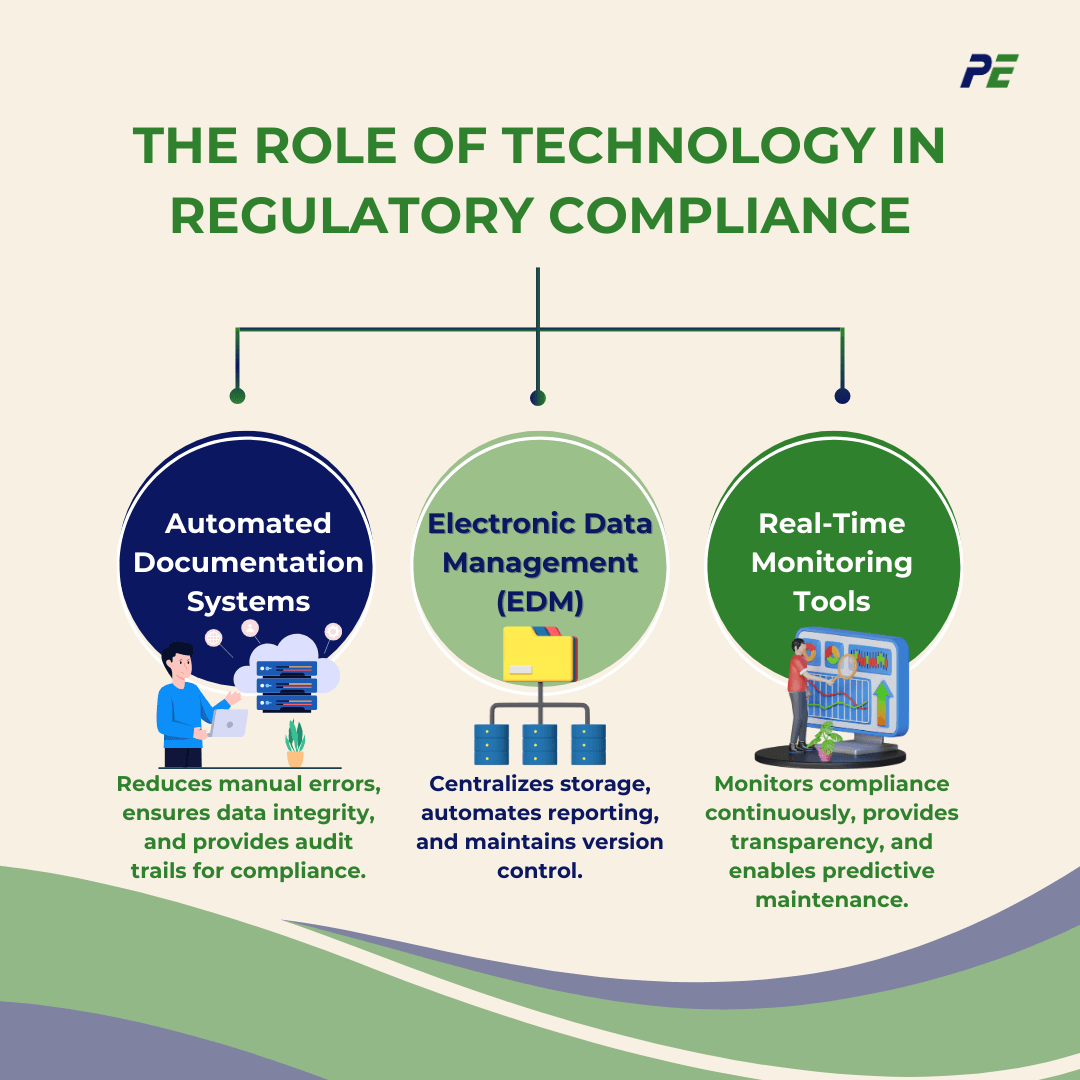
The Role of Technology in Regulatory Compliance
Technology plays a critical role in helping organizations stay compliant with regulatory changes in CQV. Here are some key technologies that support compliance in an ever-evolving regulatory landscape:
1. Automated Documentation Systems
- Eliminating Human Error: Automated systems reduce the risk of errors associated with manual documentation, which can lead to non-compliance. By streamlining documentation, companies ensure that records are accurate, complete, and consistent.
- Improved Data Integrity: Automation enforces data integrity through standardized formats and built-in checks for completeness. This ensures compliance with regulations such as FDA’s 21 CFR Part 11, which governs electronic records and signatures.
- Audit Trails: These systems create an automatic and comprehensive audit trail, making it easier to trace any changes or updates made to the records, which is crucial for compliance during inspections or audits.
2. Electronic Data Management (EDM)
- Centralized Data Storage: EDM systems enable centralized storage and easy retrieval of all documentation related to CQV. This centralization supports the consistent application of guidelines and regulations across all departments and stages of production.
- Regulatory Reporting: EDM systems can automatically generate reports that meet specific regulatory requirements, ensuring that reporting is accurate, timely, and compliant.
- Version Control: Electronic systems offer automated version control, ensuring that only the most current and approved documents are used, preventing outdated procedures from being followed.
3. Real-Time Monitoring Tools
- Continuous Compliance: Real-time monitoring tools ensure that processes and equipment are continuously monitored, providing instant alerts for deviations from pre-defined specifications or regulatory guidelines.
- Data Transparency: Real-time data collection and reporting make it easier for organizations to demonstrate continuous adherence to regulations and address potential compliance issues quickly.
- Predictive Maintenance: By tracking equipment performance, these tools predict when maintenance is required, helping prevent equipment failure and ensuring processes remain in control and compliant.
By leveraging these technologies, organizations can ensure that their CQV processes not only meet current regulatory requirements but are also adaptable to future changes.
Conclusion
In conclusion, navigating regulatory changes in CQV requires a combination of well-established best practices and agile, proactive strategies. Organizations can build a strong foundation by conducting regular audits, maintaining robust documentation practices, and providing continuous staff training. Simultaneously, they must stay responsive to regulatory updates by leveraging practical strategies such as phased technology implementation, continuous monitoring, and cross-functional collaboration.
By integrating advanced technologies like automated documentation, electronic data management, and real-time monitoring, companies can streamline CQV processes, ensure ongoing compliance, and maintain product quality in an increasingly complex regulatory environment. Remaining proactive and technologically equipped will ensure compliance and safeguard operational efficiency and long-term success.
Partnering with PharmEng Technology
Partnering with PharmEng Technology gives your organization the expert guidance and technological edge needed to stay ahead in a rapidly changing regulatory environment. Our team delivers customized solutions that blend industry best practices with innovative tools, helping you streamline CQV processes, strengthen data integrity, and adapt seamlessly to new regulations. From proactive audits to advanced documentation and real-time monitoring systems, we empower your business to navigate regulatory challenges and stay compliant confidently.
Ready to boost compliance, enhance efficiency, and protect product quality? Contact us at info.asia@pharmeng.com and let’s embark on this journey to success together!

About PharmEng Technology
PharmEng Technology is a global consulting firm specializing in pharmaceutical engineering, regulatory affairs, and compliance. With a commitment to quality and innovation, PharmEng Technology provides comprehensive solutions to meet the evolving needs of the pharmaceutical and biotechnology industries.
Contact Information
PharmEng Technology
Email: info.asia@pharmeng.com





