
IoT in CQV: Connecting Systems for Enhanced Validation
by Dhika Prameswari and Rida Hadirah Ramli
The Internet of Things (IoT) is transforming industries globally, and its impact on Commissioning, Qualification, and Validation (CQV) is particularly notable. By connecting devices, systems, and processes, IoT offers a revolutionary approach to enhancing validation efficiency, data collection, and overall compliance. This article compares traditional CQV and IoT-enabled CQV, explores its role, highlights key technologies involved, and the benefits and challenges associated with its implementation. Additionally, the regulatory considerations critical to IoT integration in CQV are discussed, offering a comprehensive view of how these technologies reshape the validation landscape.

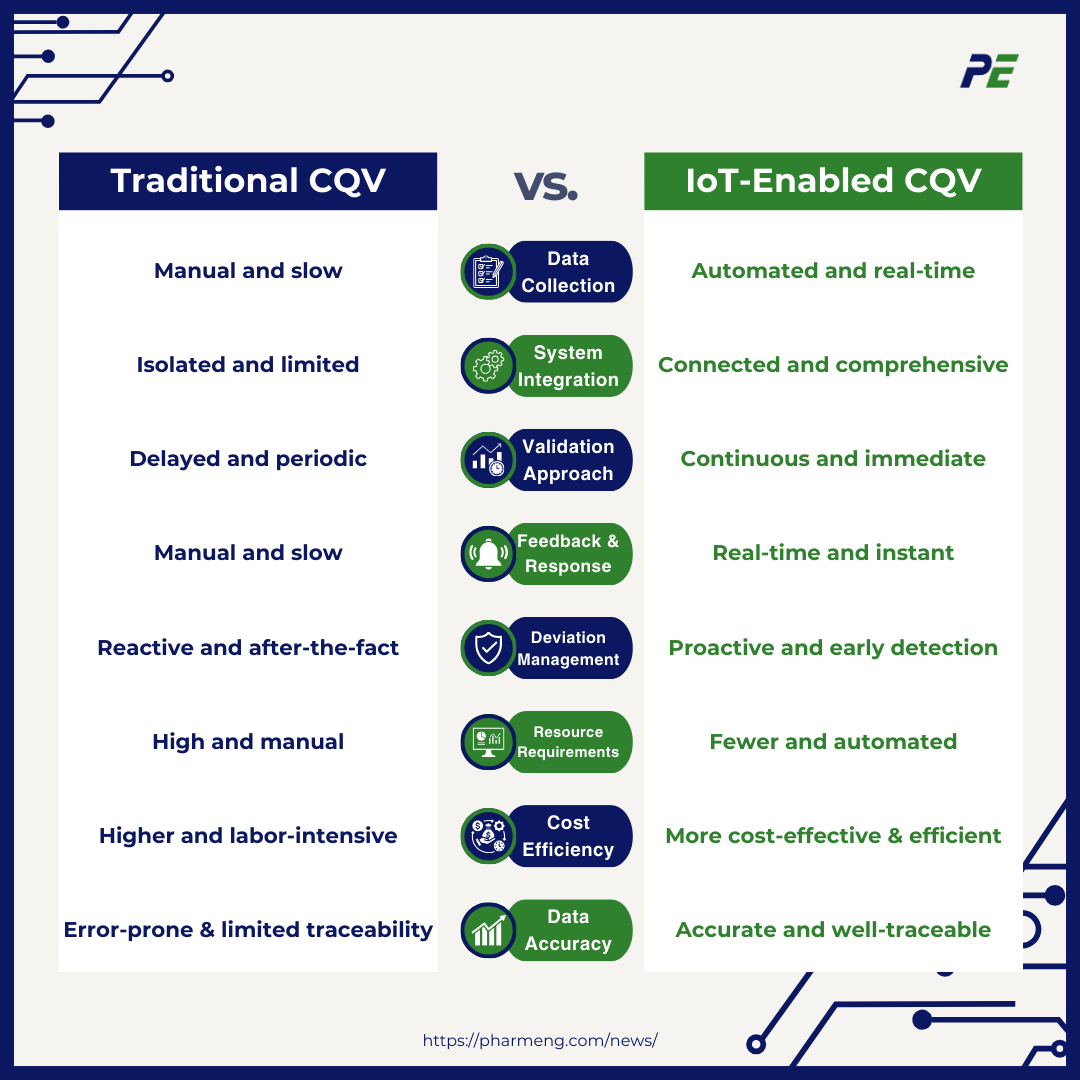
Traditional CQV vs. IoT-Enabled CQV
Here is a comparison table between traditional CQV and IoT-enabled CQV:
|
Aspect |
Traditional CQV |
IoT-Enabled CQV |
|---|---|---|
|
Data Collection |
|
|
|
System Integration |
|
|
|
Validation Approach |
|
|
|
Feedback & Response |
|
|
|
Deviation Management |
|
|
|
Resource Requirements |
|
|
|
Cost Efficiency |
|
|
|
Data Accuracy |
|
|
Overall, IoT-enabled CQV provides a more efficient, accurate, and proactive approach to validation, addressing the limitations of traditional methods and helping improve process reliability, regulatory compliance, and product quality.
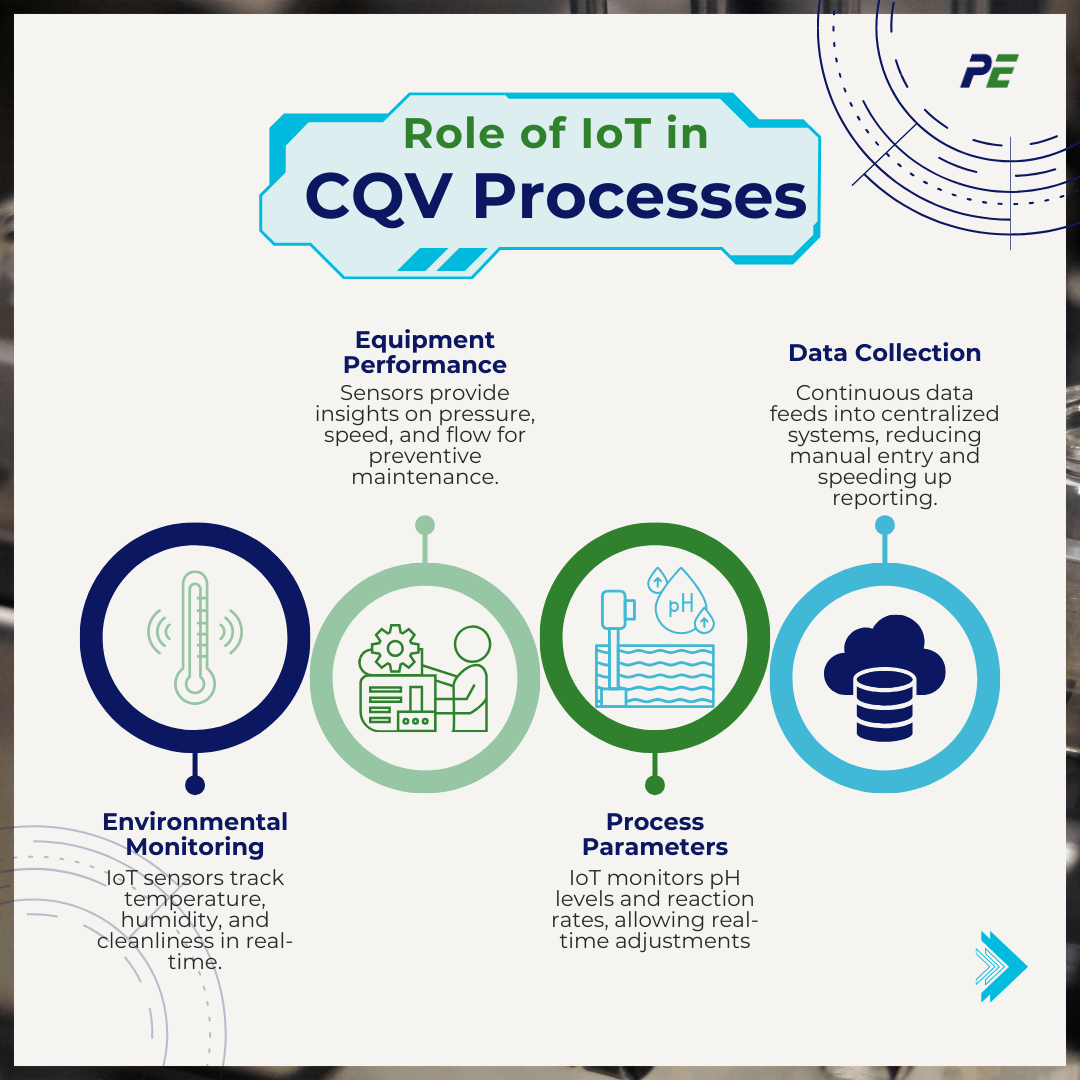
Role of IoT in CQV Processes
IoT devices and sensors play a crucial role in improving the accuracy, efficiency, and compliance of CQV processes. By offering real-time monitoring and continuous data flow, IoT enhances process optimization and predictive maintenance while also improving regulatory compliance. Here is how IoT impacts key areas in CQV:
- Monitoring Environmental Conditions
IoT sensors continuously track critical environmental parameters such as temperature, humidity, air pressure, and cleanliness in manufacturing areas.
- Monitoring Equipment Performance
Sensors monitor operational parameters like pressure, speed, and flow rate, offering insights into equipment performance and enabling preventive maintenance.
- Tracking Process Parameters
IoT systems monitor key process parameters, including pH levels and reaction rates, during production. This allows for ongoing validation and adjustment in real-time.
- Continuous Data Collection and Reporting
IoT devices enable the continuous collection of vast amounts of data, which is directly fed into centralized systems for analysis, reducing manual data entry and ensuring quicker, more accurate reporting.
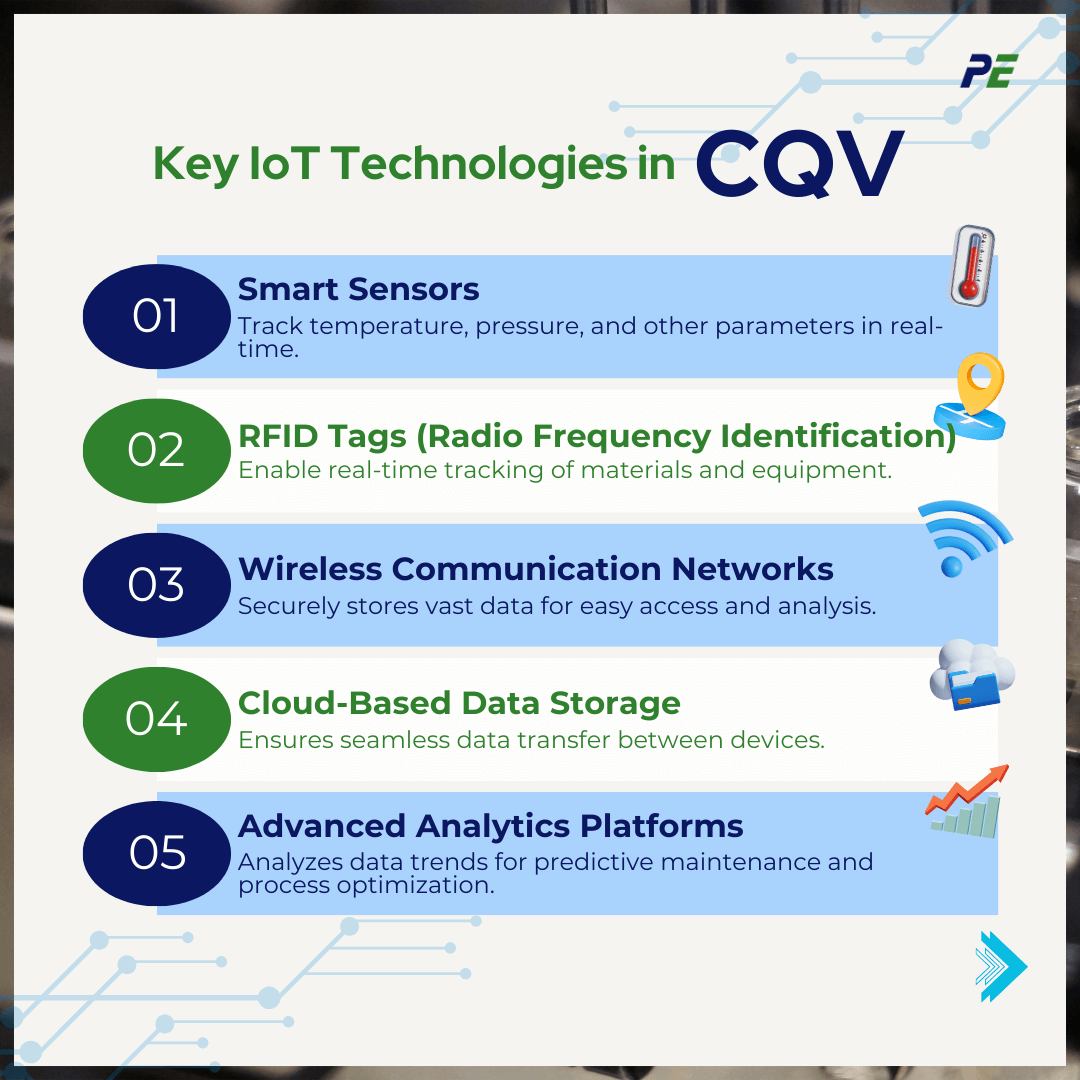
Key IoT Technologies in CQV
The integration of various IoT components ensures that validation processes are more accurate, efficient, and compliant. Here is a breakdown of the specific IoT technologies used in CQV:
- Smart Sensors
Temperature, humidity, pressure, and pH sensors are used to continuously monitor critical parameters in real-time. These sensors ensure that environmental conditions and process variables remain within validated ranges.
- RFID Tags (Radio Frequency Identification)
RFID tags allow for the tracking of materials, equipment, and products throughout the manufacturing process. These tags provide real-time data on the location and status of items, helping to streamline inventory management and ensure traceability.
- Wireless Communication Networks
IoT devices rely on wireless networks such as Wi-Fi, Bluetooth, or 5G to communicate data between sensors, devices, and central monitoring systems.
- Cloud-Based Data Storage
Cloud storage is essential for managing the vast amount of data generated by IoT devices in CQV processes. Data from smart sensors, RFID tags, and other devices is collected and stored in the cloud, ensuring easy access and scalability.
- Advanced Analytics Platforms
These platforms can identify trends, anomalies, and correlations in real time, allowing for predictive maintenance, process optimization, and quicker response to deviations.
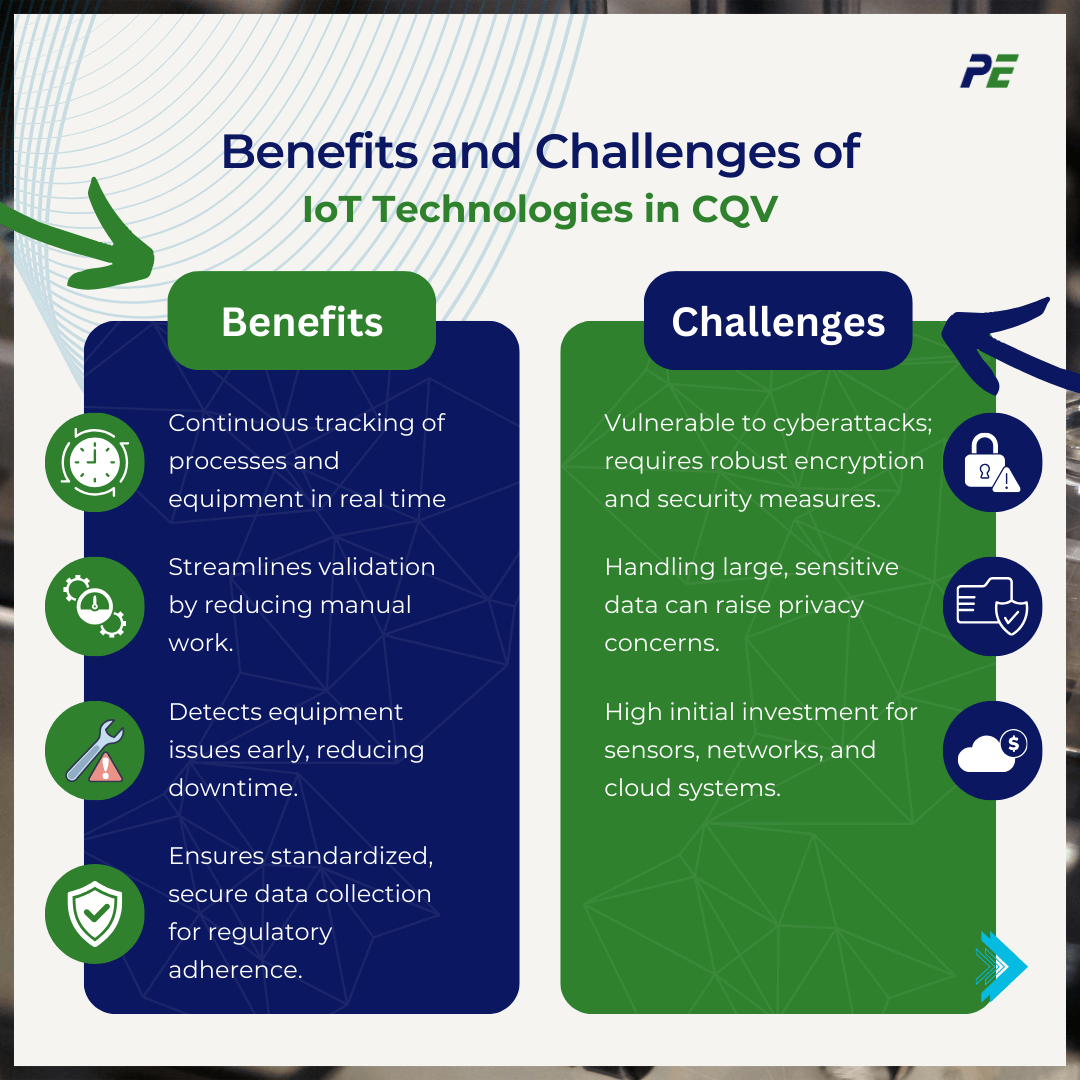
Benefits and Challenges of IoT Technologies in CQV
IoT technologies bring significant benefits to CQV but also pose various challenges. A balanced approach is essential to maximize benefits while managing these challenges effectively. Below are the benefits and challenges of IoT Technologies in CQV:
The benefits of IoT Technologies in CQV:
- Real-Time Monitoring and Data Collection
IoT devices enable continuous monitoring of critical process parameters, equipment performance, and environmental conditions in real-time.
- Enhanced Process Efficiency
IoT-enabled systems reduce manual intervention and streamline data collection, resulting in faster validation processes.
- Predictive Maintenance
IoT devices can monitor the condition of equipment and predict when maintenance is required, reducing unplanned downtime.
- Improved Compliance and Data Integrity
IoT ensures that all data is collected in a standardized and secure manner, enhancing compliance with regulatory standards and improving data integrity.
The challenges in Integrating IoT into CQV:
- Cybersecurity Risks
IoT devices and networks are vulnerable to cyberattacks, which can lead to data breaches, tampering, or loss of sensitive information. Securing IoT devices through encryption, multi-factor authentication, and regular security audits is essential for maintaining data integrity and compliance.
- Data Privacy Concern
The collection and storage of large amounts of data from IoT devices raise concerns about data privacy and confidentiality, especially when dealing with sensitive information in pharmaceutical or healthcare industries.
- Cost and Infrastructure Requirements
Implementing IoT systems requires significant investment in infrastructure, including sensors, communication networks, and cloud storage. The upfront costs can be high, particularly for small or medium-sized enterprises.
While IoT offers significant advantages in real-time monitoring, organizations looking to adopt IoT in CQV processes must carefully weigh the benefits against these challenges and invest in secure, scalable, and compliant IoT infrastructure.
Regulatory Consideration of IoT in CQV
The regulatory landscape for IoT in Commissioning, Qualification, and Validation (CQV) is becoming increasingly important as IoT technologies are integrated into pharmaceutical manufacturing processes. Here is an overview of key regulatory considerations:
- 21 CFR Part 11 Compliance (FDA): The FDA’s 21 CFR Part 11 regulation governs electronic records and electronic signatures, emphasizing the need for electronic systems to be reliable, secure, and compliant with industry standards.
- GAMP 5 Guidelines: GAMP 5 encourages a lifecycle approach to system validation, from system design to decommissioning. For IoT systems, this involves maintaining validation and compliance throughout the entire lifecycle.
- Data Integrity Expectations: Regulatory authorities require data generated by IoT systems in CQV to adhere to the ALCOA+ principles, ensuring data is captured in real-time, remains accurate, and is securely stored for future reference.
- EMA Guidelines: The EMA’s Annex 11 guidance on computerized systems highlights the importance of system validation, data security, and change control. For IoT systems used in CQV, this translates to ensuring that data collected by IoT devices remains secure and protected.
Conclusion
IoT-enabled CQV offers a significant leap forward from traditional methods, providing a proactive, data-driven approach to validation that enhances process reliability and regulatory compliance. While challenges such as cybersecurity, system integration, and cost must be addressed, organizations that strategically invest in IoT technologies can achieve significant gains in validation efficiency, accuracy, and overall product quality. As IoT continues to evolve, its role in CQV will only grow, making it an essential tool for modernizing validation processes in regulated industries.
Partnering with PharmEng Technology
Partnering with PharmEng Technology enables your organization to leverage advanced IoT solutions, enhancing the efficiency and effectiveness of your CQV processes while ensuring the highest standards of regulatory compliance. Our team of experienced professionals collaborates closely with you to implement IoT-driven systems that streamline validation, improve data integrity, and provide real-time monitoring for optimized operational performance. By integrating the latest IoT advancements, we offer a comprehensive approach to improving process reliability, reducing manual intervention, and fostering long-term cost efficiency.
We invite you to explore the tailored solutions we offer. Please contact us at info.asia@pharmeng.com, and let’s work together to achieve sustained success in your industry.

About PharmEng Technology
PharmEng Technology is a global consulting firm specializing in pharmaceutical engineering, regulatory affairs, and compliance. With a commitment to quality and innovation, PharmEng Technology provides comprehensive solutions to meet the evolving needs of the pharmaceutical and biotechnology industries.
Contact Information
PharmEng Technology
Email: info.asia@pharmeng.com





