
Benefits of CSV (Computer System Validation) in Pharma
by Roseline Tio, Dhika Prameswari, Izwan Firdaus (SEA CSV SME)
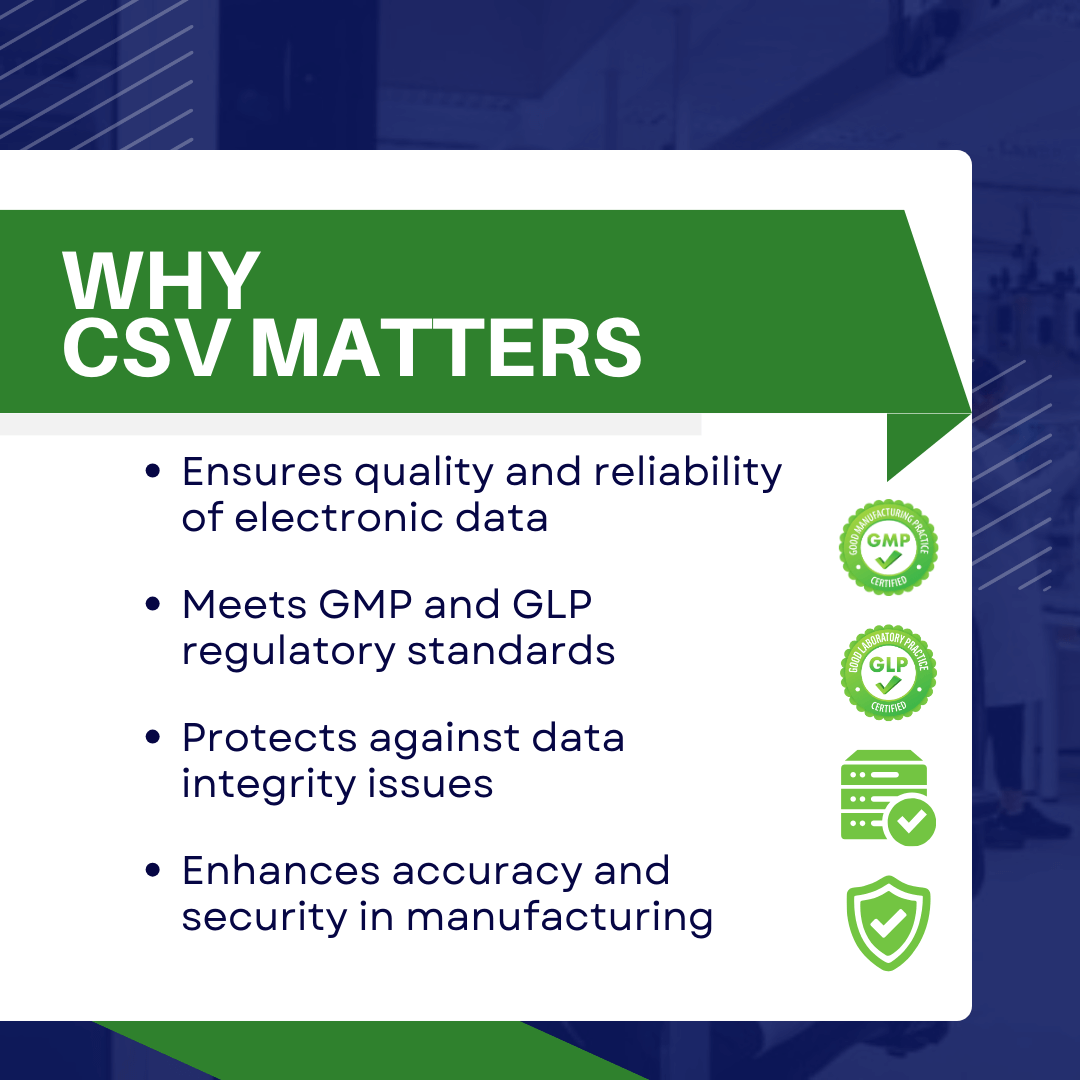
Benefits of CSV in Pharma: CSV is a brief type of computer system validation that is essential in the pharmaceutical industry for assuring the overall suitable quality control of any final product. Furthermore, CSV is a method for monitoring and validating numerous discoveries, documents, and validation methods. It helps pharmaceutical companies achieve manufacturing regulatory criteria including Good Manufacturing Practice (GMP) and Good Laboratory Practice (GLP). In addition, by adopting the CSV concept, you may be certain of the quality and dependability of electronic documents and data generated by computer systems. Aside from that, it helps to prevent data integrity violation, loss, or unauthorized access, as well as maintain the quality and accuracy of information throughout the manufacturing system’s life cycle.
Furthermore, CSV contributes to the production of high-quality pharmaceutical products by ensuring that critical processes are handled and monitored using validated computerized systems. This improves the safety and efficacy of the pharmaceutical spectrum by limiting the risk of errors throughout the manufacturing, testing, and distribution procedures. Thus, CSV involves risk evaluations to detect potential computerized system threats. Its proactive working style helps drug producers to create appropriate controls to efficiently manage hazards.
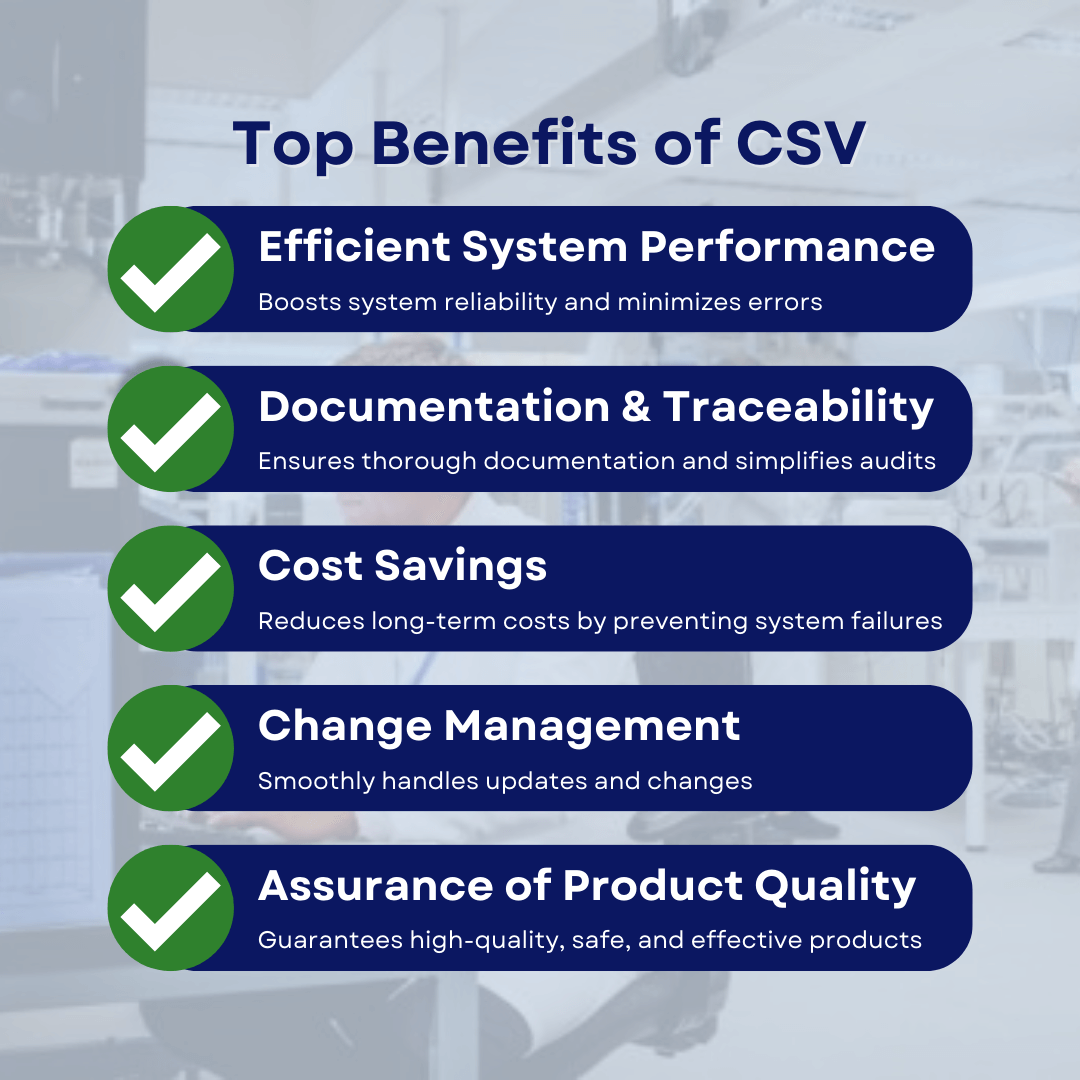
Importance of CSV in the pharmaceutical industry:
- Efficient system performance: CSV entails testing and validation efforts to verify that computerized systems work properly. This increases system dependability, lowering the risk of failures or mistakes. CSV therefore manages effective operations in important sectors such as medication manufacture and clinical trials.
- Documentation and traceability: Using CSV, pharmaceutical corporations may require detailed documentation of system requirements, design specifications, testing processes, and other pertinent information. This documentation also offers a clear record of system development and validation operations, which facilitates regulatory audits and inspections.
- Cost savings in the long run: While the initial investment in CSV processes may appear significant, keep in mind that it always yields long-term cost savings by preventing system breakdowns, regulatory noncompliance concerns, and potential recalls.
- Change management facilitation: As the pharmaceutical industry evolves, so do its operational systems and processes. As a result, to maintain compliance and data integrity, CSV requires that any modifications to computerized systems be thoroughly investigated, recorded, and confirmed.
- Assurance of product quality: One of the most significant advantages of CSV in the pharmaceutical industry. This contributes to the production of high-quality pharmaceutical products by ensuring that critical processes are managed and monitored using computerized systems. Furthermore, this technology improves product safety and efficacy by eliminating errors throughout the manufacturing, testing, and distribution processes.

Understanding the relevance of Computer System Validation (CSV) underscores its importance in the pharmaceutical industry. CSV is not just a regulatory requirement but a fundamental practice that supports the production of high-quality, safe, and effective pharmaceutical products. At PharmEng Technology, we’re passionate about elevating your pharmaceutical projects with our top-notch CSV services. Our team of skilled and certified experts is dedicated to your success. Discover how our innovative CSV solutions can drive your projects to new heights. Contact us at info.asia@pharmeng.com and let’s make excellence happen!

About PharmEng Technology
PharmEng Technology is a global consulting firm specializing in pharmaceutical engineering, regulatory affairs, and compliance. With a commitment to quality and innovation, PharmEng Technology provides comprehensive solutions to meet the evolving needs of the pharmaceutical and biotechnology industries.
Contact Information
PharmEng Technology
Email: info.asia@pharmeng.com





