Automation in the Pharmaceutical Industry: Evolution Before AI
by Dhika Prameswari and Nur Hotimah Yusof
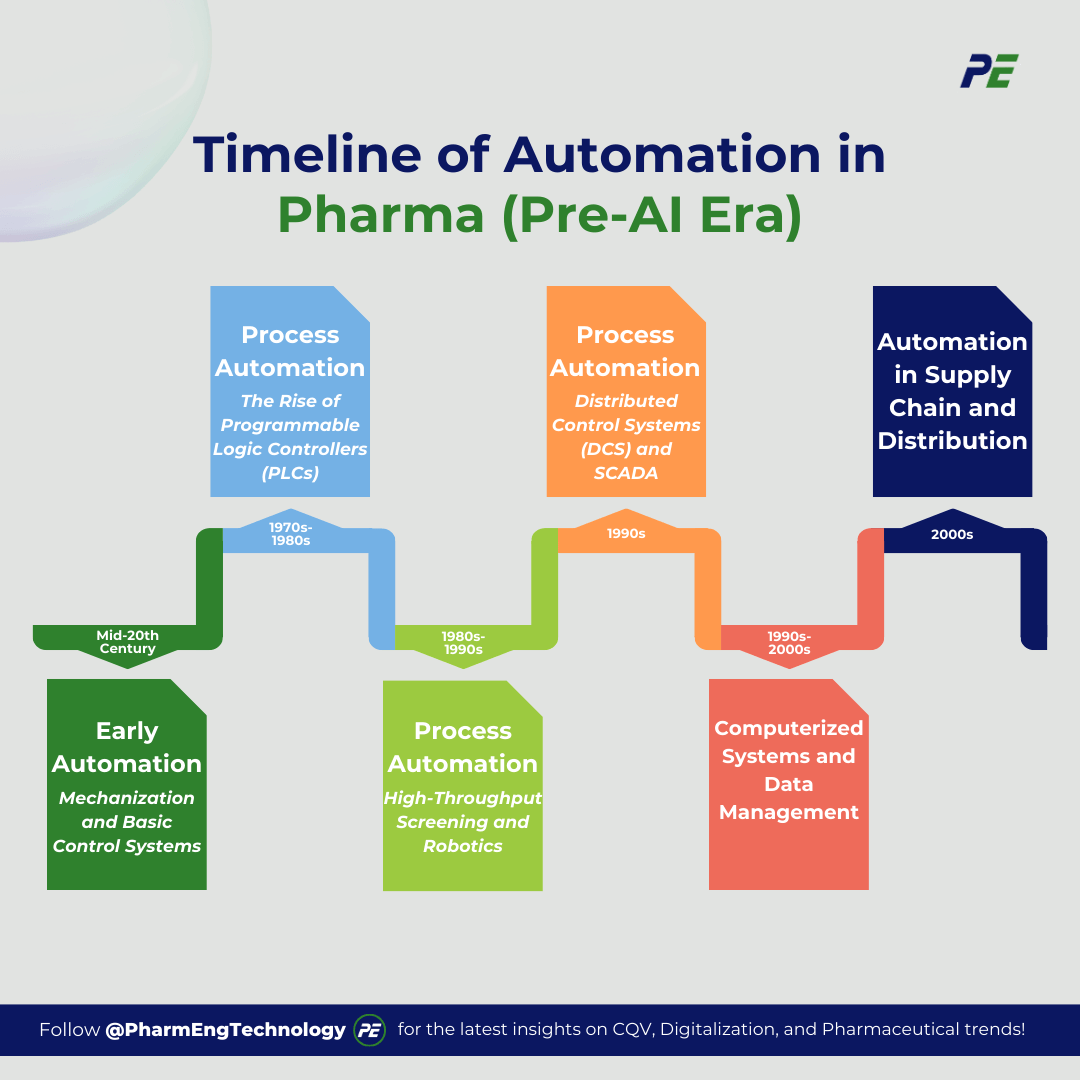
Prior to the emergence of Artificial Intelligence, the pharmaceutical sector experienced numerous phases of automation that significantly altered the processes of drug development, manufacturing, and distribution. These innovations were primarily motivated by the imperative for enhanced efficiency, accuracy, and scalability. This article explores the key stages of automation development in the industry, starting from the early century and before Al’s evolution.
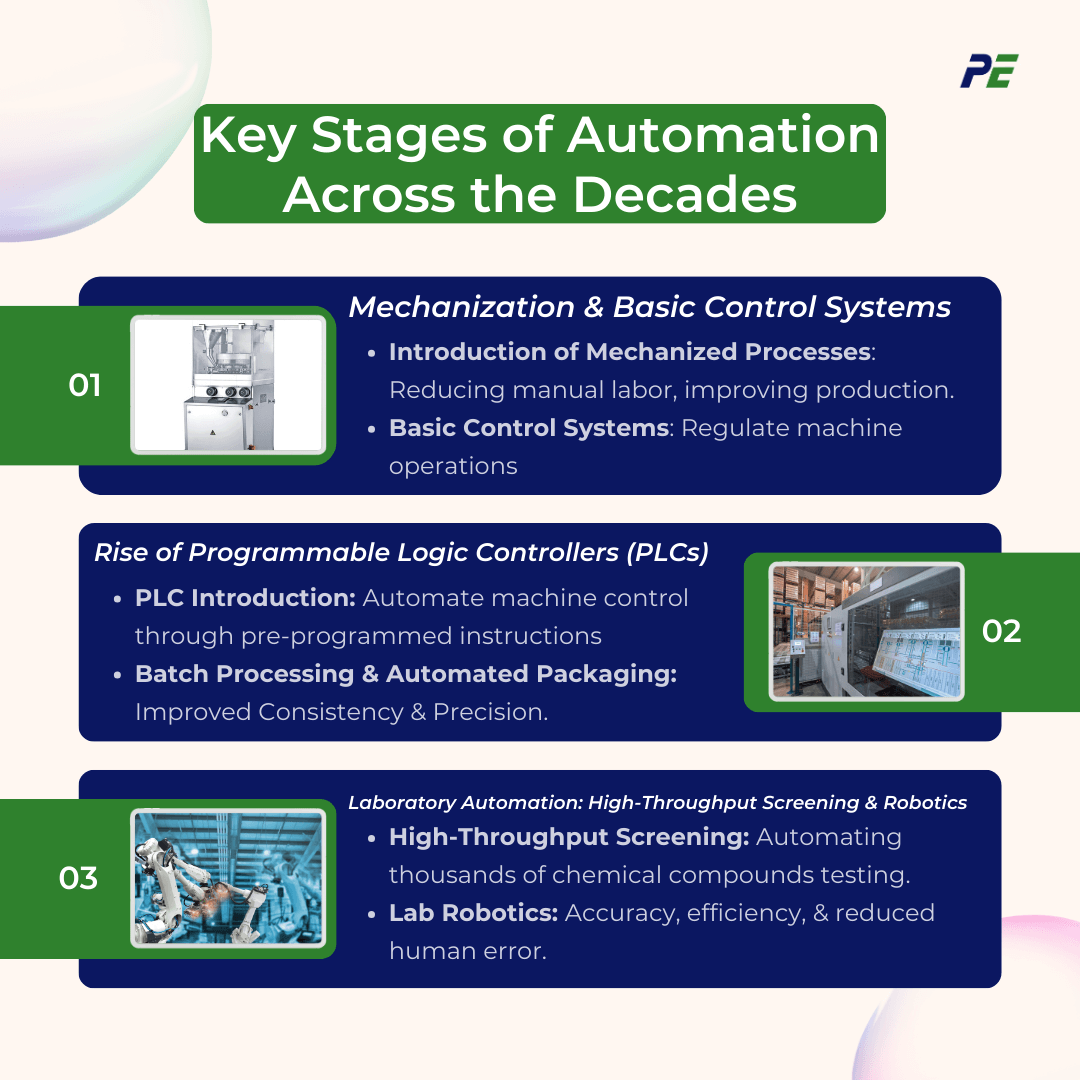
a. Early Automation: Mechanization and Basic Control Systems (Mid-20th Century)
In the mid-20th century, industry began adopting mechanized processes to streamline production.
- Introduction of Mechanized Processes: The principal characteristics of mechanization pertain to the utilization of machinery to execute functions that were formerly carried out by human labor, substantially diminishing the necessity for manual effort, enhancing production levels, and augmenting operational efficiency, uniformity, and quality oversight. Example tablet press machine.
- Basic Control Systems: Alongside mechanization, basic control systems were introduced to regulate machine operations such as timers and simple feedback loops to monitor and control processes automatically.
b. Process Automation: The Rise of Programmable Logic Controllers (PLCs) (1970s-1980s)
The decade of the 1970s and the subsequent 1980s signified a pivotal epoch in the domain of process automation characterized by the introduction of Programmable Logic Controllers (PLCs). This period represented a substantial advancement in the capacity to regulate and automate intricate industrial procedures.
- PLC Introduction: The introduction of Programmable Logic Controllers revolutionized process automation by enabling the precise control of machinery and equipment through pre-programmed instructions. Key features of PLCs (Programmable Logic Controllers) consist of programmability, real-time monitoring, and control, reliability, durability, and scalability.
- Batch Processing Automation: PLCs facilitated the management of sequential and repetitive tasks in batch production, improving consistency and efficiency. The principal characteristics are recipe management, real-time monitoring, sequential control (series of sequential steps), and data logging (record data).
- Automated Packaging Lines: Automation ensured that packaging tasks were carried out with high precision and speed, minimizing manual handling and enhancing overall production such as automated tablet packaging line and blister packaging line.
c. Laboratory Automation: High-Throughput Screening and Robotics (1980s-1990s)
The decades of the 1980s and 1990s were characterized by substantial progress in the domain of laboratory automation, propelled by ground-breaking developments including High-Throughput Screening (HTS) and robotic systems. These technological advancements profoundly altered the landscape of drug discovery and enhanced laboratory operational efficiency.
- High-Throughput Screening (HTS): High-Throughput Screening revolutionized the drug discovery process by automating the testing of thousands of chemical compounds against biological targets.
- Laboratory Robotics: By diminishing the necessity for human involvement, laboratory robotics have significantly improved accuracy, minimized human error, and enabled researchers to concentrate on more sophisticated and value-enhancing endeavors through the automation of routine tasks within laboratory settings.
- Automated Sample Storage and Retrieval: These systems improved organization, reduced the risk of sample loss or contamination, and enabled faster access to samples, thereby increasing the overall efficiency of laboratory operations.
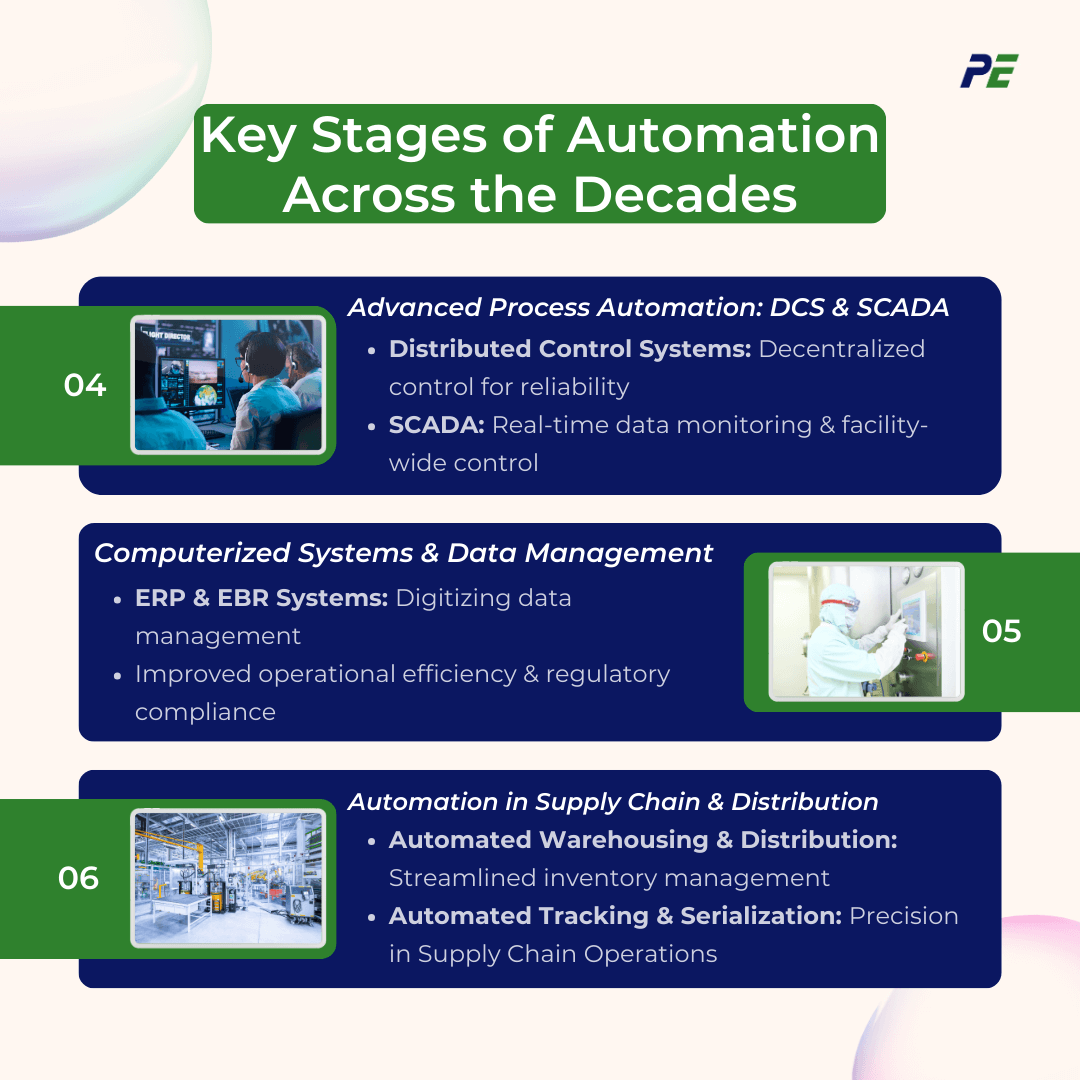
d. Advanced Process Automation: Distributed Control Systems (DCS) and SCADA (1990s)
The 1990s marked a pivotal era in advanced process automation with the development and widespread adoption of Distributed Control Systems (DCS) and Supervisory Control and Data Acquisition (SCADA) systems. These technologies revolutionized the management and monitoring of industrial processes.
- Distributed Control Systems (DCS): Distributed Control Systems introduced a sophisticated approach to managing industrial processes by decentralizing control across multiple stages with various parameters. This decentralization improved reliability and scalability, as each control unit could operate independently while still being integrated into a cohesive system.
- Supervisory Control and Data Acquisition (SCADA): SCADA systems provide a comprehensive solution for real-time data monitoring and control across entire production facilities.
e. Computerized Systems and Data Management (1990s-2000s)
This period marked the transition from manual and semi-automated data management practices to fully computerized systems. The adoption of advanced computer systems transformed how data was collected, stored, processed, and analyzed such as Enterprise Resource Planning (ERP), Electronic Batch Records (EBR), and Quality Management Systems (QMS), leading to improvements in operational efficiency, regulatory compliance, and overall quality management.
f. Automation in Supply Chain and Distribution (2000s)
In the 2000s, automation expanded beyond production to include supply chain and distribution.
- Automated Warehousing and Distribution: Automated storage and retrieval systems (ASRS) revolutionized warehousing by automating the storage and retrieval of inventory.
- Automated Tracking and Serialization: The integration of automated tracking and serialization systems brought a new level of precision to supply chain management.
Conclusion
Automation in the pharmaceutical industry has evolved significantly over the decades, transitioning from basic mechanization and control systems to sophisticated robotics, Distributed Control Systems (DCS), and Enterprise Resource Planning (ERP) frameworks. These technological progressions have established the foundation for the highly automated, data-centric industry. Nevertheless, the constraints of pre-artificial Intelligence (AI) automation—such as the absence of predictive analytics and data integration—highlighted the necessity for more intelligent systems. The recent advent of AI and machine learning technologies is now mitigating these deficiencies, thereby facilitating the emergence of a new epoch characterized by intelligent, adaptive automation within the pharmaceutical industry.
Partnering with PharmEng Technology
At PharmEng Technology, we understand the complexities of automation within the pharmaceutical industry and the importance of adapting to the ever-evolving landscape. Our expertise in implementing robust automation solutions ensures that your organization can effectively navigate the challenges of drug development, manufacturing, and distribution. By collaborating with us, you can access tailored strategies that leverage advanced technologies, streamline processes, and enhance compliance while driving operational efficiency.
Contact us via email at info.asia@pharmeng.com to learn how we can support your automation initiatives and help you stay ahead in this competitive industry.
About PharmEng Technology
PharmEng Technology is a global consulting firm specializing in pharmaceutical engineering, regulatory affairs, and compliance. With a commitment to quality and innovation, PharmEng Technology provides comprehensive solutions to meet the evolving needs of the pharmaceutical and biotechnology industries.
Contact Information
PharmEng Technology
Email: info.asia@pharmeng.com