Early Engagement with Regulatory Bodies
by Dhika Prameswari, Nur Hotimah Yusof
In the rapidly evolving landscape of the pharmaceutical and biotechnology industries, the integration of innovative technologies such as artificial intelligence (AI) is transforming development processes. However, navigating the regulatory environment can be a daunting challenge. Early engagement with regulatory bodies is a strategic approach that enables companies to align their development efforts with regulatory requirements. By fostering collaboration with regulators, organizations can ensure compliance, streamline validation practices, and ultimately enhance the likelihood of successful product approvals. This article explores the importance of early engagement with regulatory agencies and its role in shaping effective development strategies.
Understanding Regulatory Requirements
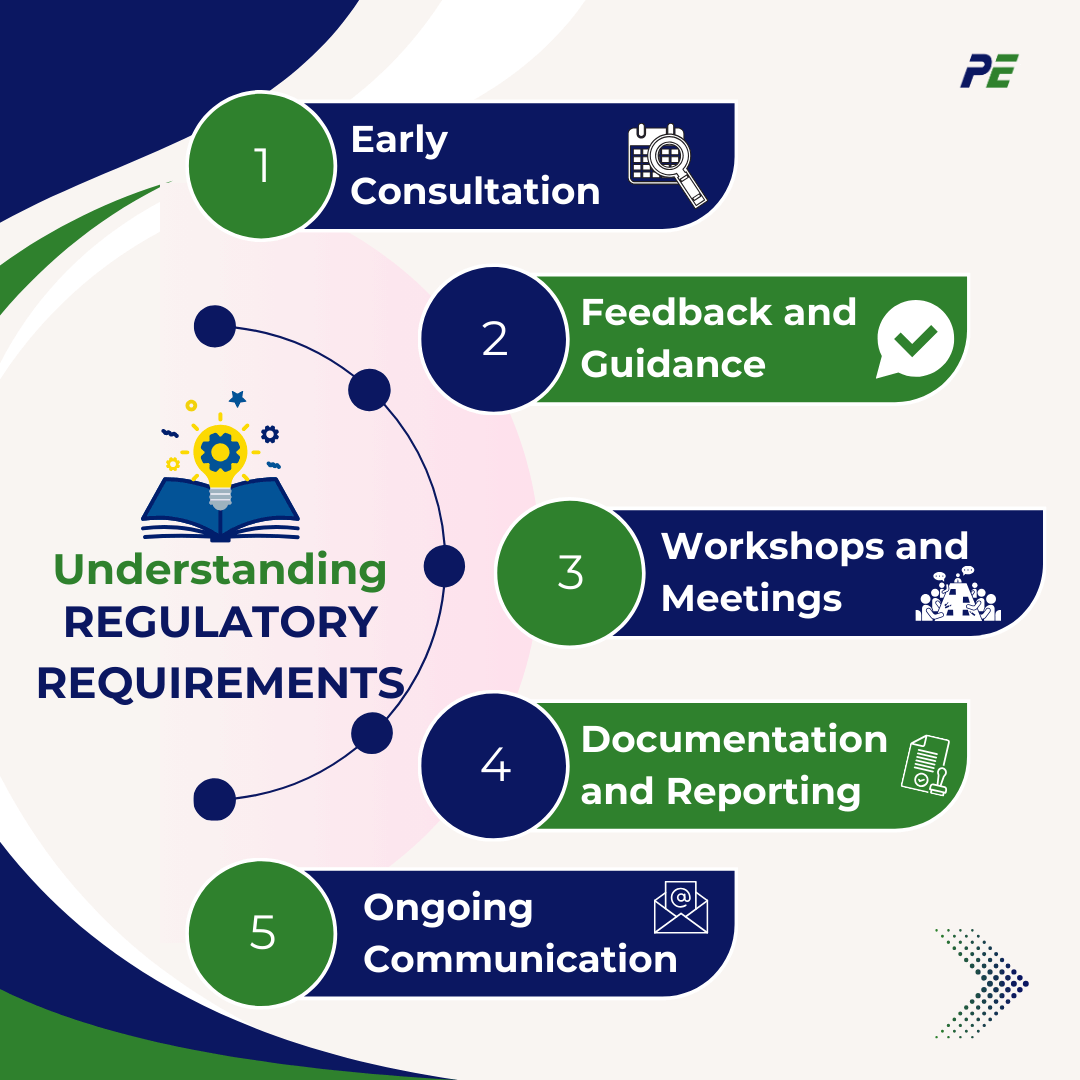
Engagement with regulatory bodies refers to the proactive communication and collaboration between organizations (such as pharmaceutical companies or technology developers) and the regulatory authorities that oversee industry standards and compliance. This engagement can take various forms, including:
- Early Consultation: Involving regulatory agencies early in the development process to ensure that planned technologies or products meet relevant regulations and standards.
- Feedback and Guidance: Seeking input from regulators on specific projects, proposals, or processes to clarify expectations and requirements.
- Workshops and Meetings: Participating in workshops, technical webinars, public meetings, or advisory committees to discuss new technologies, share best practices, and understand regulatory priorities.
- Documentation and Reporting: Preparing and submitting necessary documentation, such as pre-market submissions, to demonstrate compliance with regulations.
- Ongoing Communication: Maintaining open communication to address any emerging issues, changes in regulations, or updates on ongoing projects.
Effective engagement with regulatory bodies helps organizations align their practices with legal and ethical standards, streamline approval processes, and reduce the risk of compliance issues.
Tailoring Validation Practices to Regulatory Compliance
Regulatory bodies, such as the FDA (Food and Drug Administration) and EMA (European Medicines Agency), establish comprehensive guidelines for validation processes. These guidelines outline the necessary steps for demonstrating that products and processes meet predefined quality standards. Understanding these frameworks is crucial for organizations aiming to navigate the regulatory landscape effectively. Here are several tailored strategies to enhance validation practices:
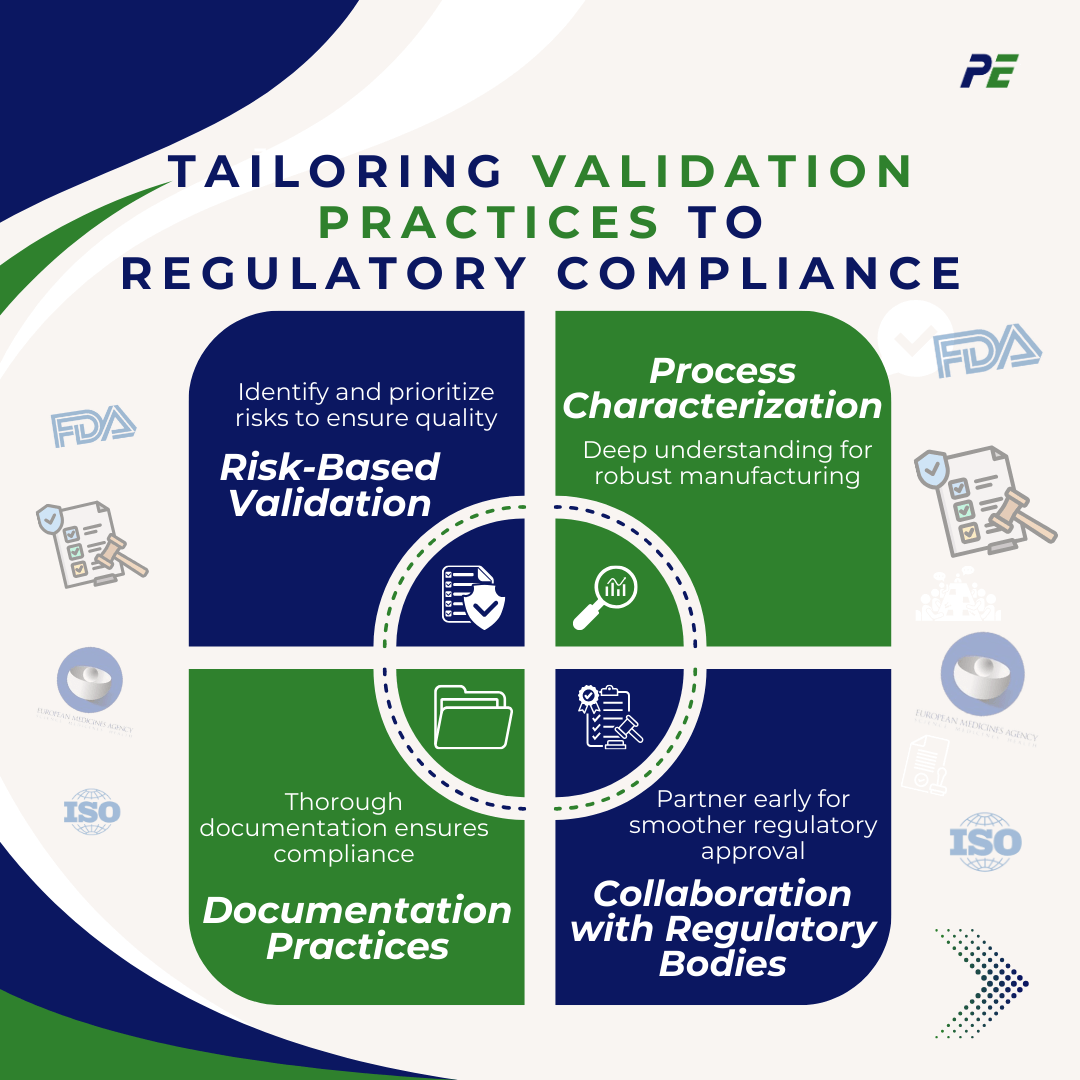
1. Risk-Based Validation focuses on identifying and prioritizing risks associated with manufacturing processes and product quality.
Implementation:
- Conduct a risk assessment to determine critical quality attributes (CQAs) and critical process parameters (CPPs).
- Use Failure Mode and Effects Analysis (FMEA) or other risk assessment approach to assess potential failure points and mitigate risks.
- Allocate resources and efforts to validate high-risk areas, ensuring that potential failures are addressed early.
2. Process Characterization, develop a deep understanding of the manufacturing process to identify and control variability.
Implementation:
- Gather data through development studies (like lab-scale and pilot-scale) or experimental investigation to establish baseline performance and initial control strategies.
- Use statistical tools or methods such as the design of experiments (DOE) to analyze process data, which informs the design of robust parameters and output.
3. Documentation Practices, ensure that all validation activities are thoroughly documented in compliance with regulatory standards.
Implementation:
- Develop comprehensive validation plans, protocols, and reports that are aligned with regulatory requirements.
- Utilize standardized and best practice templates and formats to maintain consistency and clarity in documentation.
4. Collaboration with Regulatory Bodies, engage with regulatory agencies during the early stages of the validation process to meet regulatory requirements and guidelines.
Implementation:
- Schedule pre-IND (Investigational New Drug) meetings or consultations with regulatory representatives.
- Seek feedback on validation strategies and protocols to ensure alignment with regulatory requirements.
Benefit of Tailored Validation Practices
Tailoring validation practices to meet specific regulatory and operational needs offers numerous advantages, especially in the pharmaceutical and biotechnology sectors. Here are some key benefits:
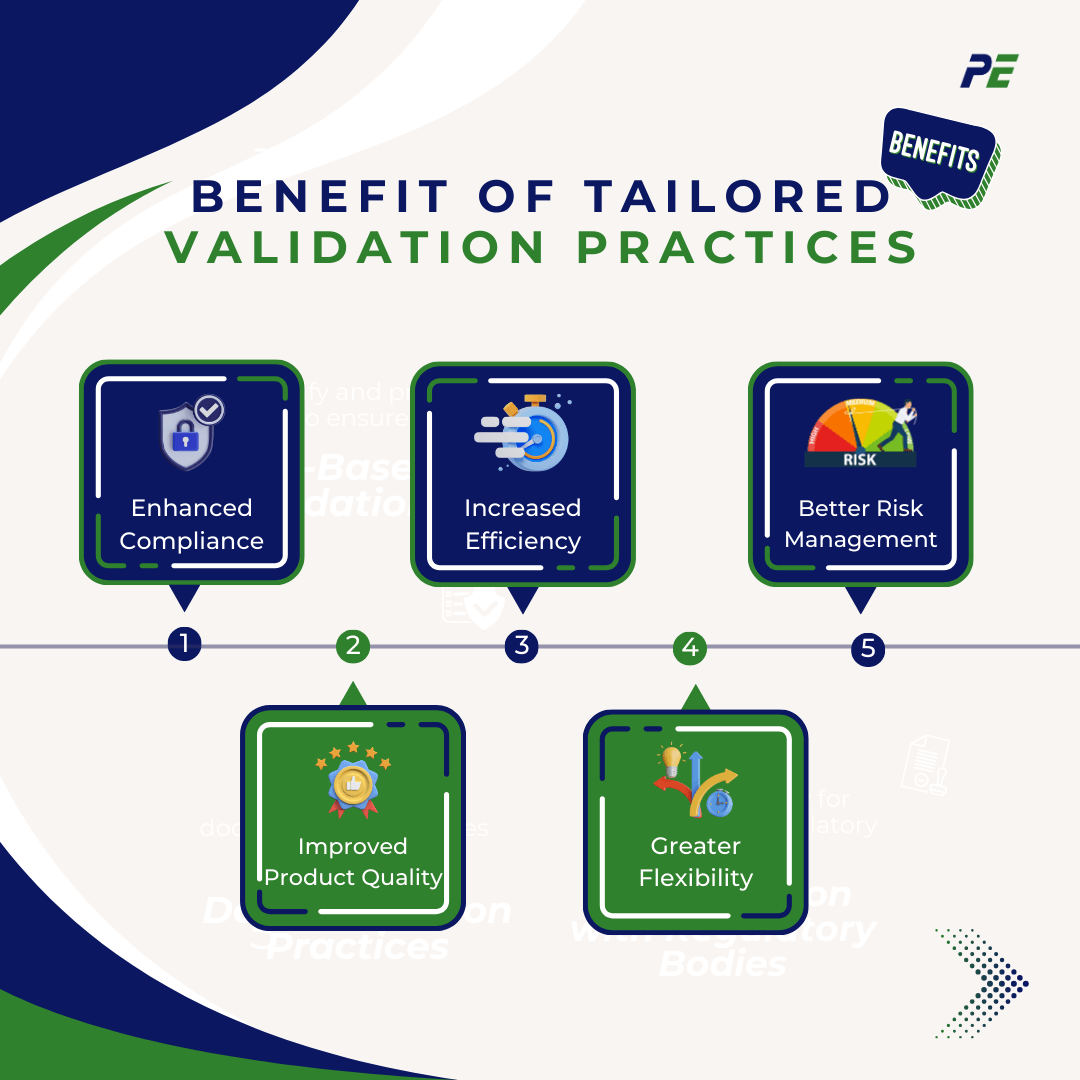
1. Enhanced Compliance
Description: Customized validation approaches ensure that processes align with regulatory requirements.
Benefit: This proactive alignment minimizes the risk of non-compliance, reducing the likelihood of regulatory penalties and delays in product approval.
2. Improved Product Quality
Description: Focused validation efforts on critical areas lead to a better understanding of processes and potential risks.
Benefit: By systematically addressing these risks, organizations can significantly improve product quality, thereby resulting in safer products.
3. Increased Efficiency
Description: Tailored validation methodologies enhance operational efficiencies by allocating resources to areas of greatest necessity.
Benefit: This methodology has the potential to diminish superfluous validation procedures, thereby conserving both temporal and material resources, whilst accelerating the timeline for market introduction.
4. Greater Flexibility
Description: Customized validation strategies allow organizations to adapt their practices based on specific project requirements and regulatory updates.
Benefit: This flexibility enables quicker responses to changing regulatory landscapes and evolving technology, ensuring that validation practices remain relevant and effective.
5. Better Risk Management
Description: Tailored validation practices focus on identifying and mitigating specific risks associated with manufacturing and testing processes.
Benefit: Elevated risk management practices result in a diminished occurrence of product failures, recalls, or regulatory compliance challenges, hence cultivating confidence in the organization’s products.
Tailored validation methodologies are essential for fulfilling regulatory obligations while simultaneously ensuring the quality and safety of products. Through the adaptation of these practices, organizations can attain compliance, enhance operational efficiency, and foster a culture of continuous improvement.
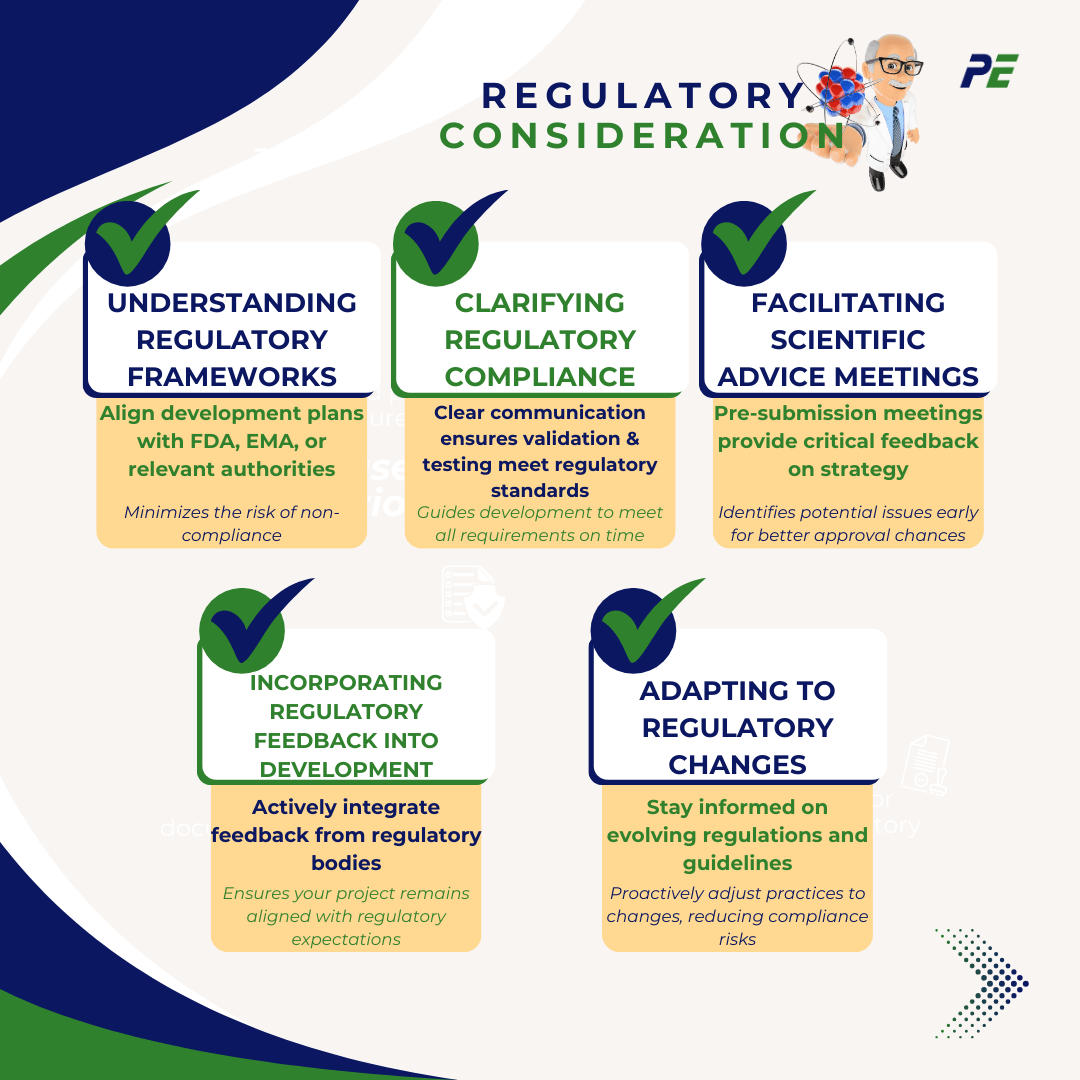
Regulatory Consideration
Engaging with regulatory bodies early in the development process is crucial for ensuring compliance and successful product approval in the pharmaceutical and biotechnology industries. Here are key regulatory considerations related to this practice:
1. Understanding Regulatory Frameworks
Overview: Familiarize with the specific regulations that govern the industry, such as FDA, EMA, or other relevant authorities.
Importance: Early engagement helps ensure that development plans align with the necessary regulatory frameworks and guidelines, hence reducing the risk of non-compliance.
2. Clarifying Regulatory Compliance
Overview: Direct communication with regulatory agencies provides clarity on their expectations for documentation, validation, and testing.
Importance: Comprehending these anticipations can guide the development process, ensuring that all requisite requirements are met within an appropriate timeframe.
3. Facilitating Scientific Advice Meetings
Overview: Seeking scientific advice or pre-submission meetings with regulatory bodies can provide invaluable feedback for development strategy.
Importance: These meetings identify potential issues early, allowing for adjustments that enhance the likelihood of regulatory approval.
4. Incorporating Regulatory Feedback into Development
Overview: Actively integrating feedback from regulatory bodies into your project plans and validation practices.
Importance: This iterative process leads to better-aligned practices and documentation, making compliance more straightforward.
5. Adapting to Regulatory Changes
Overview: Keep updated with the evolving regulations and guidelines that may impact the development process.
Importance: Early engagement allows organizations to adapt their practices proactively, rather than reactively, to any changes in the regulatory landscape.
Early engagement with regulatory bodies is a proactive strategy that significantly enhances compliance and streamlines the product development process. By understanding regulatory frameworks, clarifying expectations, and fostering collaboration, organizations can navigate the complexities of regulatory requirements more effectively, leading to successful product approvals and improved outcomes in the pharmaceutical industry.
Conclusion
In conclusion, early engagement with regulatory bodies is a critical component of successful product development in the pharmaceutical and biotechnology sectors. By establishing great communication and fostering collaborative relationships, organizations will have a better understanding of regulatory expectations, identify compliance risks, and adapt their validation practices accordingly. This proactive approach not only minimizes the risk of delays but also enhances the overall efficiency of the development process. As the industry continues to evolve, embracing early engagement with regulators will be essential for driving innovation while ensuring the safety and efficacy of new products.
Partnering with PharmEng Technology
Partner with PharmEng Technology and gain a competitive edge in navigating regulatory complexities. Our team specializes in crafting tailored validation strategies that align with regulatory guidelines, minimize compliance risks, and enhance your product quality—ultimately fast-tracking your approval process. Whether you’re developing cutting-edge pharmaceuticals or innovative biotech solutions, early regulatory engagement is key to avoiding costly delays and ensuring the smoothest path to market.
Are you ready to streamline your development and validation practices? Request a free, no-obligation assessment with our experts to uncover gaps, optimize processes, and ensure compliance with the latest FDA and EMA requirements. Don’t let regulatory hurdles slow you down—contact us today at info.asia@pharmeng.com or fill out the form here to start driving your success forward. Let PharmEng Technology be your trusted partner in achieving faster, more efficient regulatory approvals!
About PharmEng Technology
PharmEng Technology is a global consulting firm specializing in pharmaceutical engineering, regulatory affairs, and compliance. With a commitment to quality and innovation, PharmEng Technology provides comprehensive solutions to meet the evolving needs of the pharmaceutical and biotechnology industries.
Contact Information
PharmEng Technology
Email: info.asia@pharmeng.com