The Future of CQV: Trends Shaping the Pharmaceutical Industry Part 1
by Dhika Prameswari, Rida Hadirah Ramli, Nur Syahirah Mustafa
As the pharmaceutical industry continues to evolve, the integration of emerging technologies is transforming Commissioning, Qualification, and Validation (CQV) processes. Innovations such as automation, digitalization, and real-time data monitoring are not only streamlining validation efforts but also enhancing compliance and product quality. In parallel, regulatory agencies are adapting their frameworks to emphasize risk-based, efficient approaches that align with these technological advancements. This article explores how CQV is redefined by these trends, focusing on improving efficiency, accuracy, and regulatory adherence in the pharmaceutical sector.
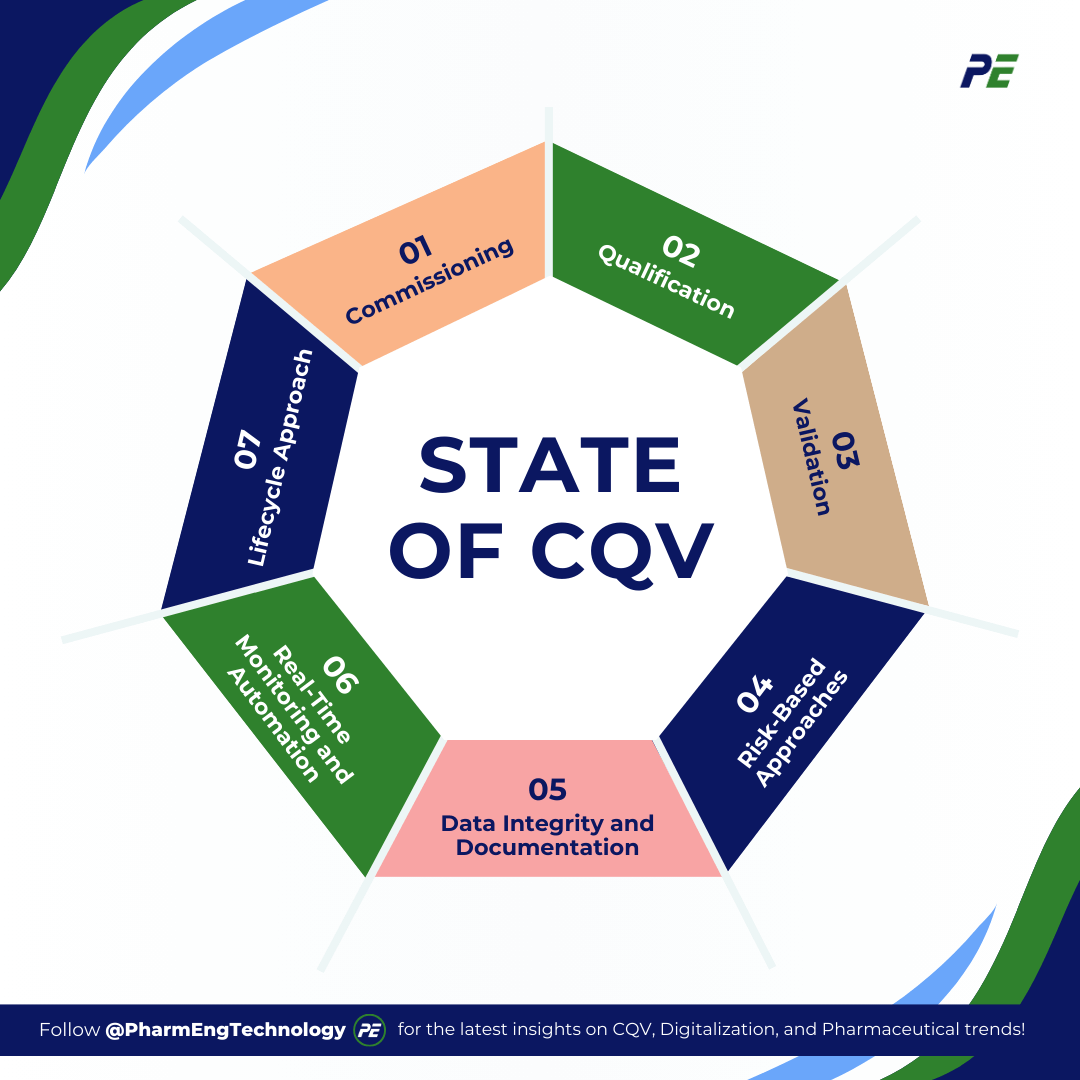
State of CQV
In today’s pharmaceutical industry, CQV processes are critical in ensuring that facilities, equipment, and processes meet regulatory standards while maintaining operational efficiency. These processes are essential for maintaining product quality, patient safety, and compliance with Good Manufacturing Practices (GMP). The CQV framework typically follows a structured, multi-phase approach, with key methodologies evolving as new trends emerge. Below are the primary phases and their role in maintaining compliance and quality:
- Commissioning: This initial phase focuses on the planning, installation, and functional testing of equipment and systems to ensure they meet design specifications. It involves critical tasks such as verifying utilities, ensuring proper installation, and confirming that all components operate as intended before moving on to qualification.
- Qualification: Divided into several stages (Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ)), qualification ensures that systems and equipment consistently perform within predetermined parameters. Each stage involves detailed protocols to validate that equipment is fit for use.
- Validation: Validation focuses on ensuring that processes, once qualified, produce consistent, reliable outcomes that meet pre-established criteria. Validation is critical for maintaining compliance with standards such as GMP. The validation process is ongoing and requires constant monitoring and review.
- Risk-Based Approaches: CQV methodologies are increasingly incorporating risk management tools, such as Failure Mode and Effects Analysis (FMEA) to prioritize activities based on their impact on product quality and patient safety. This approach optimizes resources and improves overall efficiency.
- Data Integrity and Documentation: Current practices emphasize the importance of data integrity in CQV, with a focus on ensuring accuracy, consistency, and traceability of data throughout the lifecycle of equipment and processes. Robust documentation is maintained to ensure compliance with regulatory requirements like 21 CFR Part 11 for electronic records.
- Real-Time Monitoring and Automation: Advanced technologies, such as IoT devices, SCADA systems, and automated data capture tools, are increasingly used to enhance real-time monitoring and control, enabling continuous verification of critical process parameters.
- Lifecycle Approach: A modern shift toward a lifecycle approach ensures that CQV is not a one-time event but an ongoing process, continuously verifying system performance through real-time data and ongoing qualification efforts throughout the equipment’s operational life.
These methodologies represent the foundation of CQV, ensuring alignment with regulatory expectations while improving operational efficiency and product quality. However, as the industry moves toward greater integration of digital technologies, the future of CQV promises even more transformation.
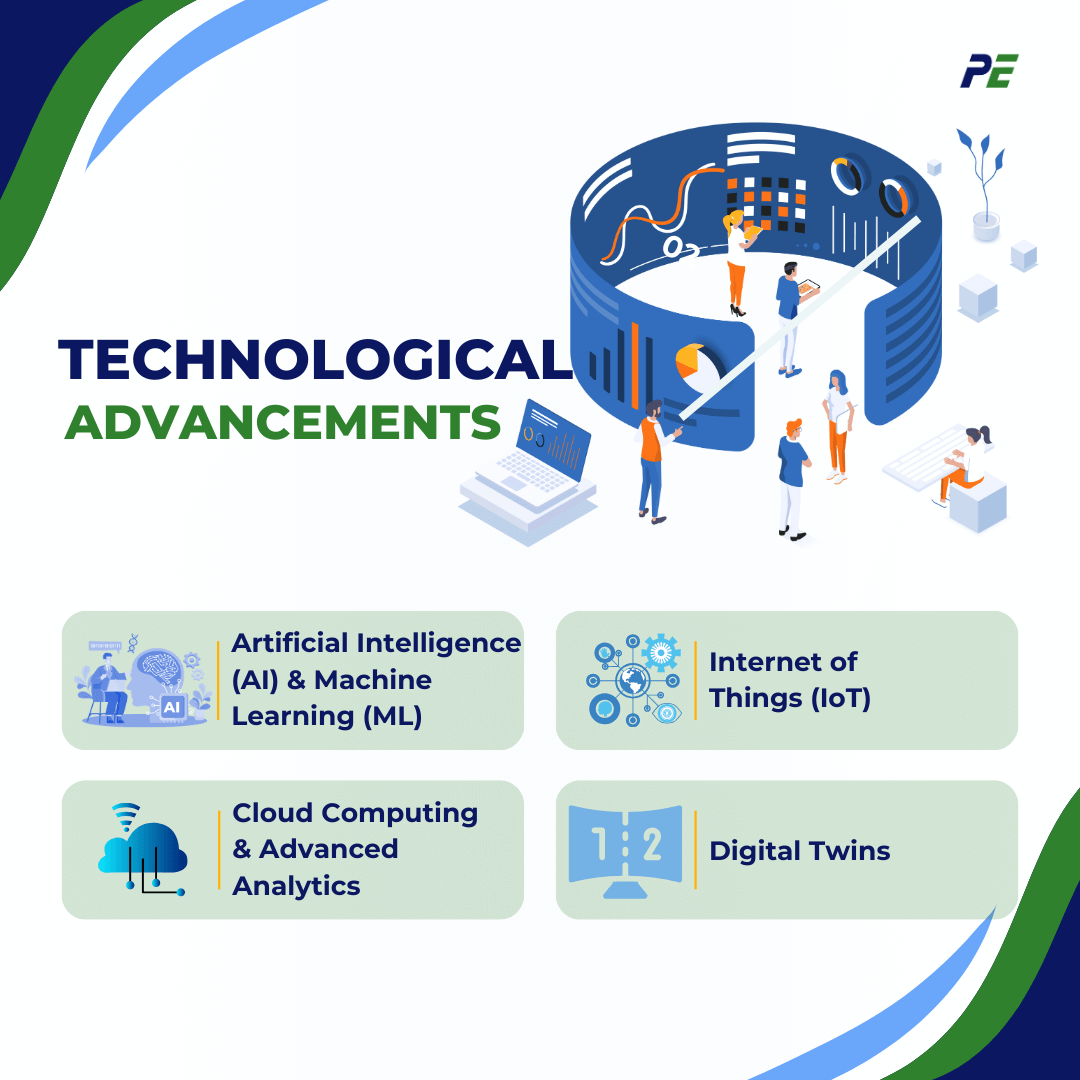
Technological Advancements
The pharmaceutical industry is entering a new era of CQV, driven by cutting-edge technologies such as Artificial Intelligence (AI), Machine Learning (ML), and the Internet of Things (IoT). These advancements offer advanced capabilities that enhance data accuracy, streamline operations, and improve decision-making, ultimately driving efficiency and compliance.
1. Artificial Intelligence (AI) and Machine Learning (ML)
AI and ML are revolutionizing how data is analyzed and how processes are optimized in CQV. Their impact includes:
- Predictive Maintenance: AI-driven predictive models can analyze equipment data to anticipate maintenance needs, reducing unplanned downtime and ensuring that equipment remains reliable throughout qualification and validation.
- Enhanced Data Analysis: AI can rapidly analyze large datasets collected during validation, identifying trends, anomalies, and deviations that may be overlooked during manual reviews. This leads to faster decision-making and more precise adjustments during the CQV process.
- Continuous Improvement ML algorithms learn from historical data, enabling systems to adapt and optimize over time. This fosters continuous improvement, particularly in process validation and performance monitoring.
2. Internet of Things (IoT)
IoT technology provides real-time data through interconnected devices and sensors, improving the ability to monitor and control processes in CQV. The benefits of IoT include:
- Real-Time Monitoring: IoT-enabled sensors continuously monitor critical process parameters such as temperature, humidity, pressure, and equipment performance, providing real-time insights into equipment and process performance.
- Enhanced Data Traceability: IoT devices automatically capture and store data, ensuring an accurate and tamper-proof record of all events. This enhances data integrity and compliance with regulatory standards.
- Improved Process Control: IoT allows for continuous process adjustments based on real-time data, improving control and consistency in product quality.
3. Cloud Computing and Advanced Analytics
Cloud-based systems are increasingly used to store and manage the vast amount of data generated during CQV processes. Advanced analytics tools then help interpret this data, providing insights for continuous process improvement.
- Scalability and Accessibility: Cloud computing allows for the easy storage and retrieval of data, enabling remote access and collaboration across teams.
- Integration with AI and ML: Cloud platforms can integrate with AI and ML tools to provide real-time insights, predictive analytics, and more sophisticated data analysis, enhancing the overall validation process.
4. Digital Twins
Digital twin technology is a game-changer in CQV. By creating virtual replicas of physical systems, companies can simulate CQV processes before real-world implementation.
- Simulation and Optimization: By simulating equipment performance and process conditions, digital twins help to predict how the systems will behave under various conditions.
- Risk Mitigation: This technology reduces the risk of errors during actual operations by allowing for extensive testing in a virtual environment before implementation.
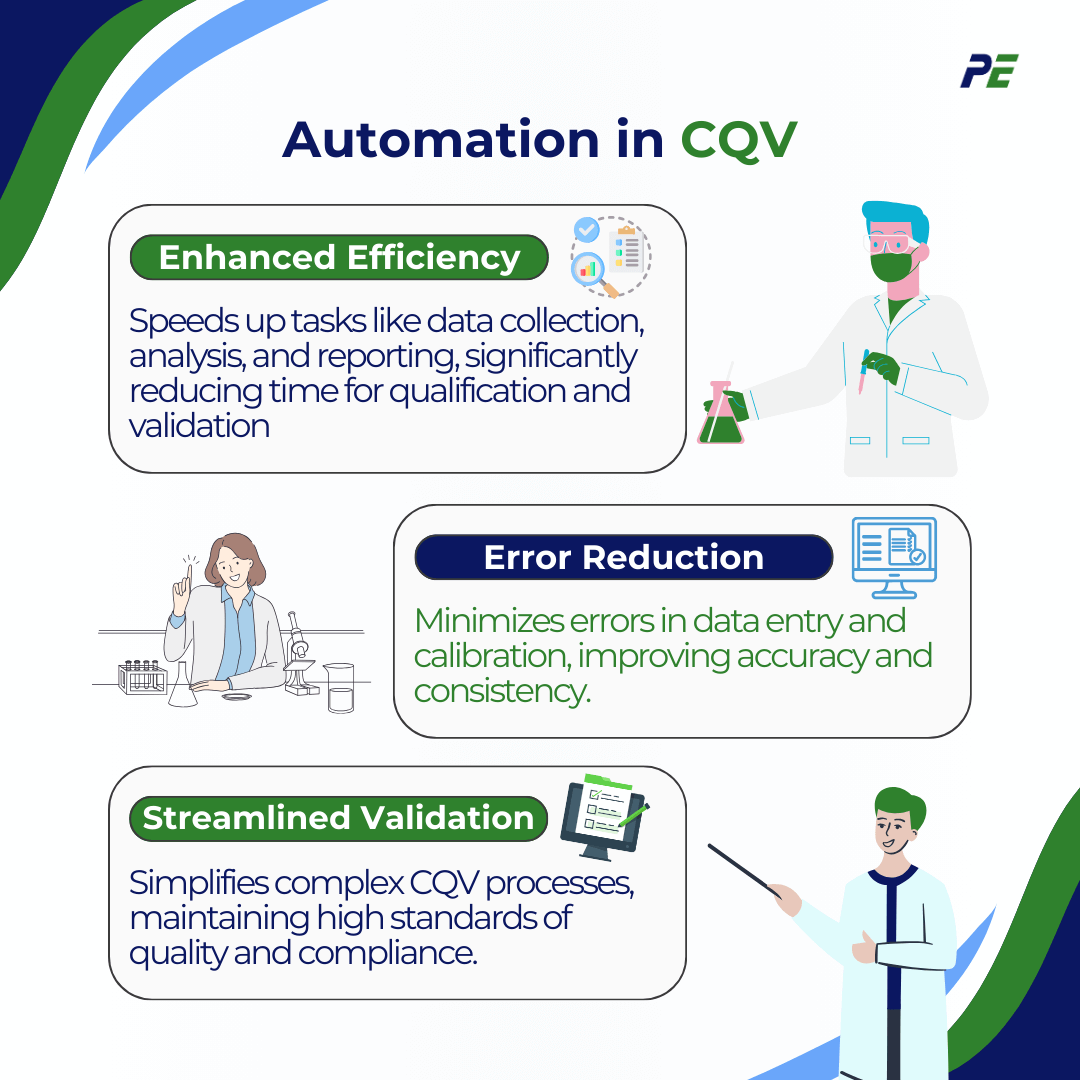
Automation in CQV
Automation is playing a central role in the evolution of CQV, particularly through the integration of AI and IoT technologies. Automated data capture and analysis reduces human intervention, minimizing errors while ensuring compliance with data integrity standards. As pharmaceutical companies work to meet stringent regulatory standards while optimizing efficiency, automation is transforming CQV processes. Key impacts include:
- Enhanced Efficiency: Automation speeds up time-consuming tasks like data collection, analysis, reporting, and system monitoring, significantly reducing the time needed for qualification and validation.
- Error Reduction: Manual CQV processes are prone to human error in areas like data entry and equipment calibration. Automation minimizes these risks, improving accuracy and consistency.
- Streamlined Validation: Automation simplifies complex CQV processes, facilitating large-scale validation efforts while maintaining high standards of quality and compliance.
Incorporating automation into CQV processes is reshaping how the pharmaceutical industry achieves compliance, quality, and efficiency.
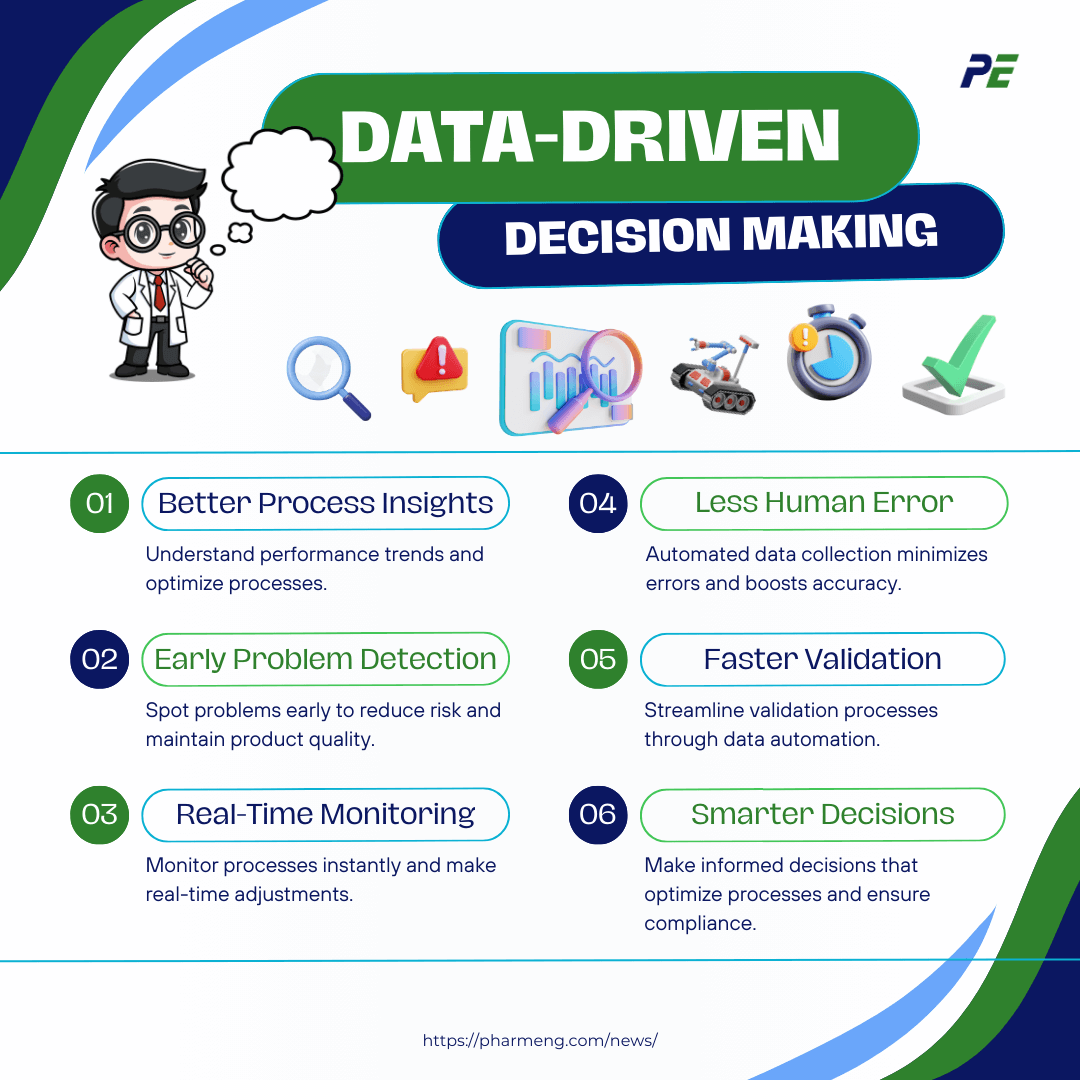
Data-Driven Decision Making
Data analytics and big data are becoming integral to CQV processes as the pharmaceutical industry generates vast amounts of data during production. Their growing importance can be highlighted through the following key points:
- Better Process Insights: Data analytics helps companies understand how their processes are performing and where improvements are needed.
- Early Problem Detection: Big data helps spot issues early, reducing the risk of failures and ensuring product quality.
- Real-Time Monitoring: With real-time data analytics, companies can adjust CQV processes instantly to keep things running smoothly.
- Less Human Error: Automated data collection minimizes manual errors, increasing the accuracy and reliability of CQV efforts.
- Faster Validation: Analytics tools streamline validation processes by automating data analysis and reducing the time required for manual reviews.
- Smarter Decisions: Data-driven insights enable pharmaceutical companies to make more informed, strategic decisions, optimizing processes while ensuring regulatory compliance.
Data analytics and big data are revolutionizing CQV, offering the potential for enhanced process control, proactive decision-making, and optimized validation strategies. By integrating these technologies, companies can ensure greater compliance, improved efficiency, and more effective use of resources.
Conclusion
In conclusion, the future of CQV in the pharmaceutical industry is being shaped by emerging technologies. These innovations are revolutionizing traditional CQV processes, enhancing efficiency, reducing human errors, and providing real-time insights for better decision-making. As the industry continues to evolve, embracing these technologies will be essential for maintaining compliance, improving process validation, and ensuring product quality. Companies that stay ahead of these trends will not only streamline their operations but also meet the growing demands of a highly regulated industry.
Partnering with PharmEng Technology
As the pharmaceutical landscape transforms, staying ahead of CQV trends is crucial for ensuring compliance, improving efficiency, and maintaining product quality. PharmEng Technology is here to help you navigate this evolving terrain with cutting-edge solutions tailored to your needs.
Partner with us to leverage the power of automation, AI, IoT, and data-driven insights in your CQV processes. Contact us at info.asia@pharmeng.com or fill out the form here for a FREE consultation. Let’s shape the future of pharmaceutical excellence together!

About PharmEng Technology
PharmEng Technology is a global consulting firm specializing in pharmaceutical engineering, regulatory affairs, and compliance. With a commitment to quality and innovation, PharmEng Technology provides comprehensive solutions to meet the evolving needs of the pharmaceutical and biotechnology industries.
Contact Information
PharmEng Technology
Email: info.asia@pharmeng.com





