Overcoming CQV Challenges: Strategies for Effective Implementation
by Dhika Prameswari and Rida Hadirah Ramli
In the dynamic field of pharmaceutical manufacturing, Commissioning, Qualification, and Validation (CQV) processes play a crucial role in ensuring that systems and equipment meet stringent quality and regulatory standards. However, implementing effective CQV can be fraught with challenges, from navigating complex regulations to managing technical and operational hurdles. As the industry evolves and technologies advance, these challenges become more intricate, requiring innovative strategies and solutions.
This article explores the common CQV challenges and provides actionable strategies for overcoming them. We will delve into issues such as adapting to new technologies, addressing regulatory complexities, and managing cross-functional teams. By understanding these challenges and implementing effective strategies, organizations can enhance their CQV processes, ensuring product quality and compliance while driving operational efficiency.
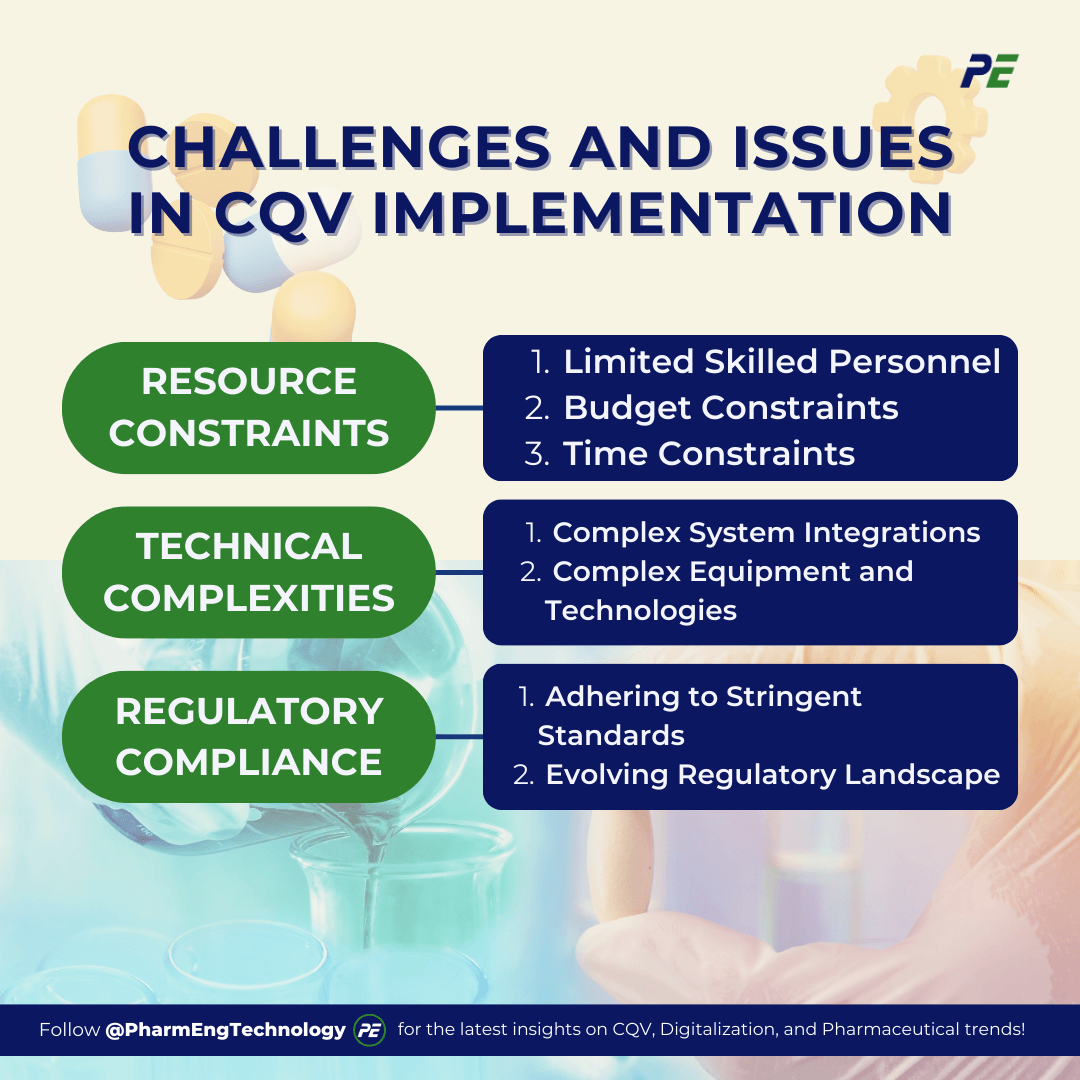
Challenges and Issues in CQV Implementation
Here are the typical challenges encountered during CQV processes:
1. Resource Constraints
- Limited Skilled Personnel
- Problem: A shortage of skilled personnel during CQV implementation.
- Impact: Increased error rates, extended project timelines, decreased quality and compliance risks, and overburdened workforce.
- Solution: Comprehensive training program, cross-training staff, leveraging external expertise, and automating routine tasks.
- Budget Constraints
- Problem: Inadequate financial resources or poor budget planning during the CQV process.
- Impact: Compromised quality, delays in timelines, increased risk of rework, and potential non-compliance.
- Solution: Prioritize critical activities, optimize resource allocation, and leverage automation tools to maximize efficiency.
- Time Constraints
- Problem: During the CQV process, tight project deadlines can create significant pressure to complete validation tasks quickly. This constraint often arises due to aggressive timelines for product launches, regulatory approvals, or production schedules.
- Impact: Time constraints can lead to rushed validations, increased error rates, insufficient documentation, and potential oversight of critical quality attributes.
- Solution: Prioritize tasks, use automation tools, promote cross-functional collaboration, and allocate resources effectively to meet deadlines without sacrificing quality.
2. Technical Complexities
- Complex System Integrations
- Problem: In CQV implementation, integrating multiple complex systems such as manufacturing execution systems (MES) and automation systems.
- Impact: Extended project timelines, increased risk of errors, higher costs, and data integrity risks.
- Solution: Thorough pre-implementation planning, standardized communication protocols, and cross-disciplinary expertise.
- Complex Equipment and Technologies
- Problem: The complexity of these tools can make installation, qualification, and validation more difficult due to their intricate functionalities and advanced operational requirements.
- Impact: Extended validation timelines, increased risk of non-compliance, and higher costs.
- Solution: Detailed validation planning, vendor engagement vendors, and tailored training programs.
3. Regulatory Compliance
- Adhering to Stringent Standards
- Problem: In CQV processes, adherence to stringent regulatory standards (such as cGMP, FDA, EMA, and other regional standards) can be challenging.
- Impact: Delays in project timelines, increased costs, and resource constraints.
- Solution: Develop a robust VMP (Validation Master Plan), regular audits and inspections, training, and skill development.
- Evolving Regulatory Landscape
- Problem: Pharmaceutical and life sciences industries face constant regulatory changes, making it challenging to maintain CQV processes and ensure compliance with new standards.
- Impact: Compliance risk, increased costs, and delays in product launches.
- Solution: Dedicated regulatory team or consultant, risk-based approach, flexible validation protocols.
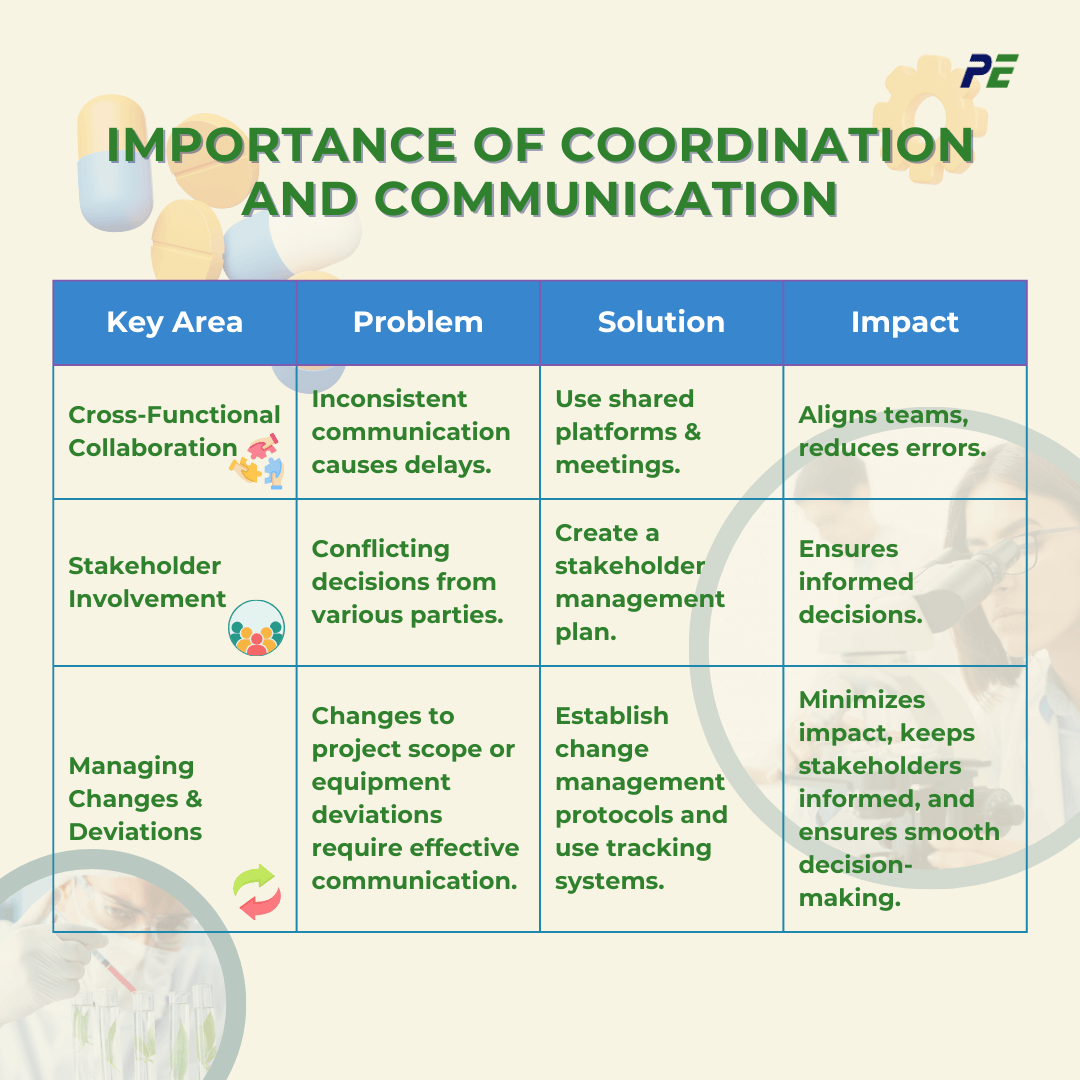
Importance of Coordination and Communication
Effective coordination and communication are vital to the success of CQV processes, as they involve multiple teams and stakeholders. Clear communication ensures alignment, prevents delays, and reduces the risk of errors during validation activities. Below are the key areas where improving communication and collaboration can help address common CQV challenges.
1. Enhancing Cross-Functional Collaboration
- Problem: CQV processes often involve multiple teams such as production, quality assurance, and regulatory affairs. Inconsistent communication or lack of clear roles can lead to confusion, delays, and even mistakes in executing validation tasks.
- Solution: Establish clear communication channels, shared platforms, and regular meetings to facilitate information sharing and collaboration.
- Impact: Effective collaboration helps in sharing critical information, addressing issues promptly, and coordinating efforts across different functional areas.
2. Coordinating Stakeholder Involvement
- Problem: CQV processes require input and approval from various stakeholders, including internal departments and external partners (e.g., equipment vendors, consultants, etc).
- Solution: Develop a stakeholder management plan that identifies key stakeholders, their roles, and communication needs. Schedule regular updates and feedback sessions to keep stakeholders informed and engaged.
- Impact: Coordinated stakeholder involvement ensures that all perspectives are considered, and decisions are made based on comprehensive information. It helps manage expectations and address potential conflicts early in the process.
3. Managing Changes and Deviation
- Problem: Changes to project scope or deviation in the processes or equipment can occur during CQV, and managing these issues requires effective communication and coordination.
- Solution: Establish a change management process that includes communication protocols for notifying and involving relevant stakeholders. Tracking systems can be used to monitor changes and deviations and their impact on the project.
- Impact: Proactive management of changes and deviations helps in minimizing their impact on the CQV process. Effective communication ensures that all relevant parties are informed and involved in decision-making.
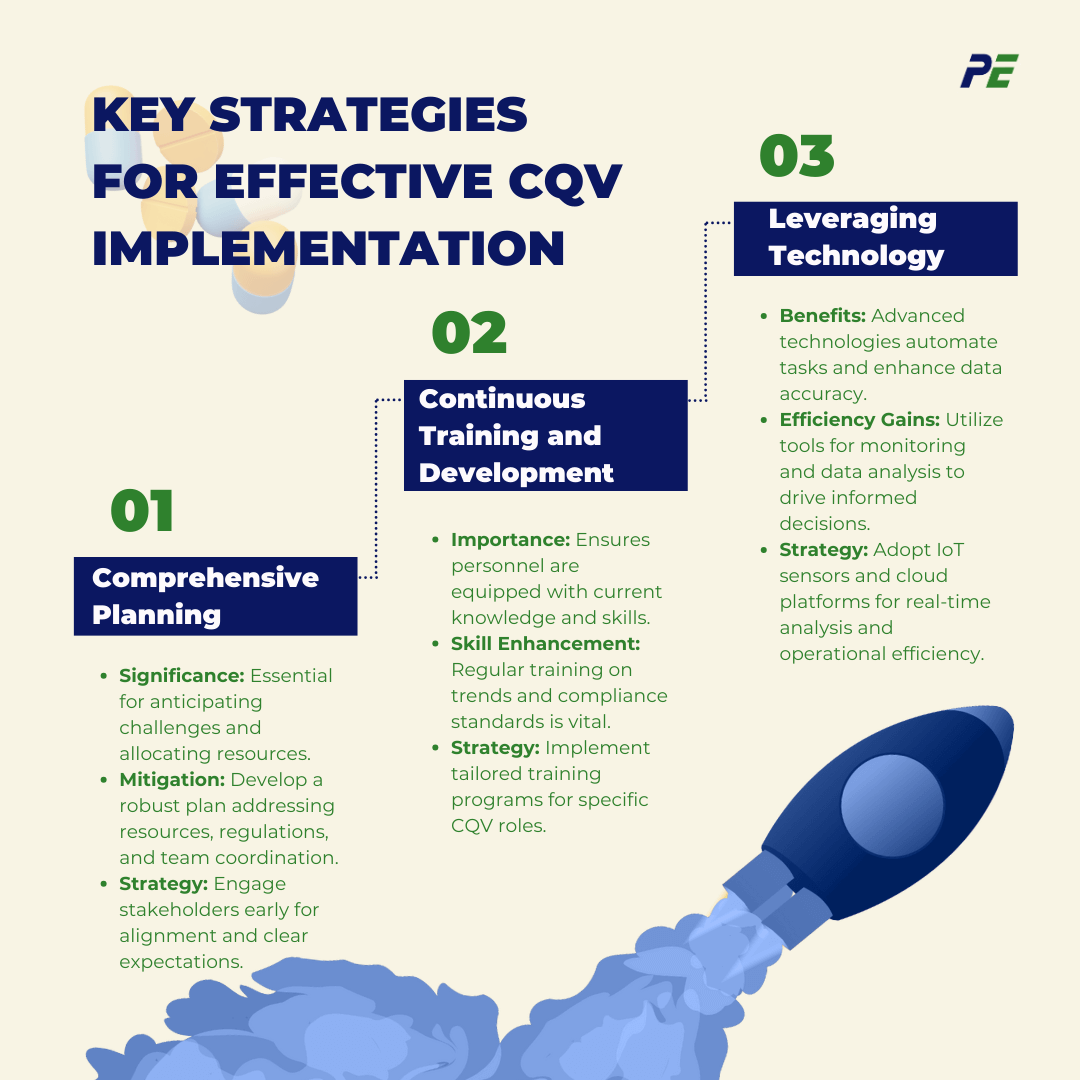
Strategies for Effective Implementation
1. Comprehensive Planning:
- Significance: Detailed and well-structured planning is critical for the success of CQV processes. It helps in anticipating potential roadblocks and efficiently allocating resources. This includes setting clear goals, timelines, and outlining the necessary steps for each stage of CQV.
- Mitigating Challenges: A robust CQV plan addresses key aspects such as resource needs, regulatory requirements, process complexities, and coordination among teams. This proactive approach allows teams to foresee challenges, avoid delays, and minimize risks.
- Strategy: Engage all relevant stakeholders early in the planning phase to ensure alignment and clarity in expectations.
2. Continuous Training and Development:
- Importance: Personnel involved in CQV processes require up-to-date knowledge and skills to handle the complexities of modern systems and regulatory requirements.
- Skill Enhancement: As technologies and regulations evolve, regular training programs are essential to keep personnel informed of the latest trends, best practices, and compliance standards.
- Strategy: Implement structured training programs that are tailored to the specific roles and responsibilities of CQV personnel to ensure that they stay informed and prepared to handle the challenges of modern CQV processes.
3. Leveraging Technology:
- Benefits: Advanced technologies, such as IoT devices, SCADA systems, and cloud-based platforms, can significantly streamline CQV processes by automating tasks, improving data accuracy, and providing real-time insights.
- Efficiency Gains: By utilizing tools for continuous monitoring, data collection, and analysis, teams can make quicker, data-driven decisions and improve process control.
- Strategy: Adopt advanced CQV technologies such as IoT sensors, automated data collection, and cloud-based platforms for real-time data analysis and reporting to improve operational efficiency and accuracy.
Conclusion
Comprehensive planning, continuous training, and the strategic use of technology are key strategies for overcoming CQV challenges. Detailed planning allows teams to anticipate risks and allocate resources efficiently, while ongoing training ensures that personnel remain skilled and knowledgeable. Leveraging advanced technologies provides real-time insights and automates complex tasks, improving the overall efficiency and reliability of CQV processes. By implementing these strategies, organizations can ensure smooth CQV execution, meet regulatory standards, and improve product quality and operational performance.
Partnering with PharmEng Technology
At PharmEng Technology, we offer a comprehensive and strategic approach to addressing the complexities of CQV implementation. With our extensive experience in the pharmaceutical and life sciences sectors, we provide end-to-end solutions, including meticulous planning, advanced technical training, and the integration of cutting-edge technologies. Our team of experts partners with organizations to navigate evolving regulatory requirements, optimize operational processes, and ensure compliance with industry standards. By engaging PharmEng Technology, your organization will benefit from a streamlined CQV process, reduced risk, and enhanced operational and quality performance.
To learn more about how PharmEng Technology can support your CQV initiatives, contact us at info.asia@pharmeng.com and let us help you drive success in your pharmaceutical manufacturing operations.
About PharmEng Technology
PharmEng Technology is a global consulting firm specializing in pharmaceutical engineering, regulatory affairs, and compliance. With a commitment to quality and innovation, PharmEng Technology provides comprehensive solutions to meet the evolving needs of the pharmaceutical and biotechnology industries.
Contact Information
PharmEng Technology
Email: info.asia@pharmeng.com