
Real-Time CQV: Monitoring and Verification in Real-Time
by Dhika Prameswari and Rida Hadirah Ramli
The need for continuous improvement and efficiency has never been more critical in the rapidly evolving landscape of pharmaceutical manufacturing. Traditional Commissioning, Qualification, and Validation (CQV) methods rely on retrospective analysis, which can delay the detection and correction of issues. This delay can lead to inefficiencies, increased risk of non-compliance, and costly rework. A key innovation addressing this need is Real-Time CQV. This approach leverages advanced technologies and methodologies to monitor, verify, and ensure the quality and compliance of processes and systems as they occur. This shift from reactive to proactive quality management ensures compliance and improves overall process efficiency.
What is Real-Time CQV?
Real-time CQV refers to the dynamic process of continuously monitoring and validating critical manufacturing operations in real-time. This approach integrates real-time data acquisition, processing, and analysis to ensure that all aspects of production, from equipment qualification to final product validation, are continuously controlled and verified. It allows for immediate detection and correction of deviations, reducing the risk of non-compliance enhancing overall process efficiency, and supporting regulatory expectations such as those outlined in ICH Q8 (Pharmaceutical Development) and ICH Q10 (Pharmaceutical Quality Systems).

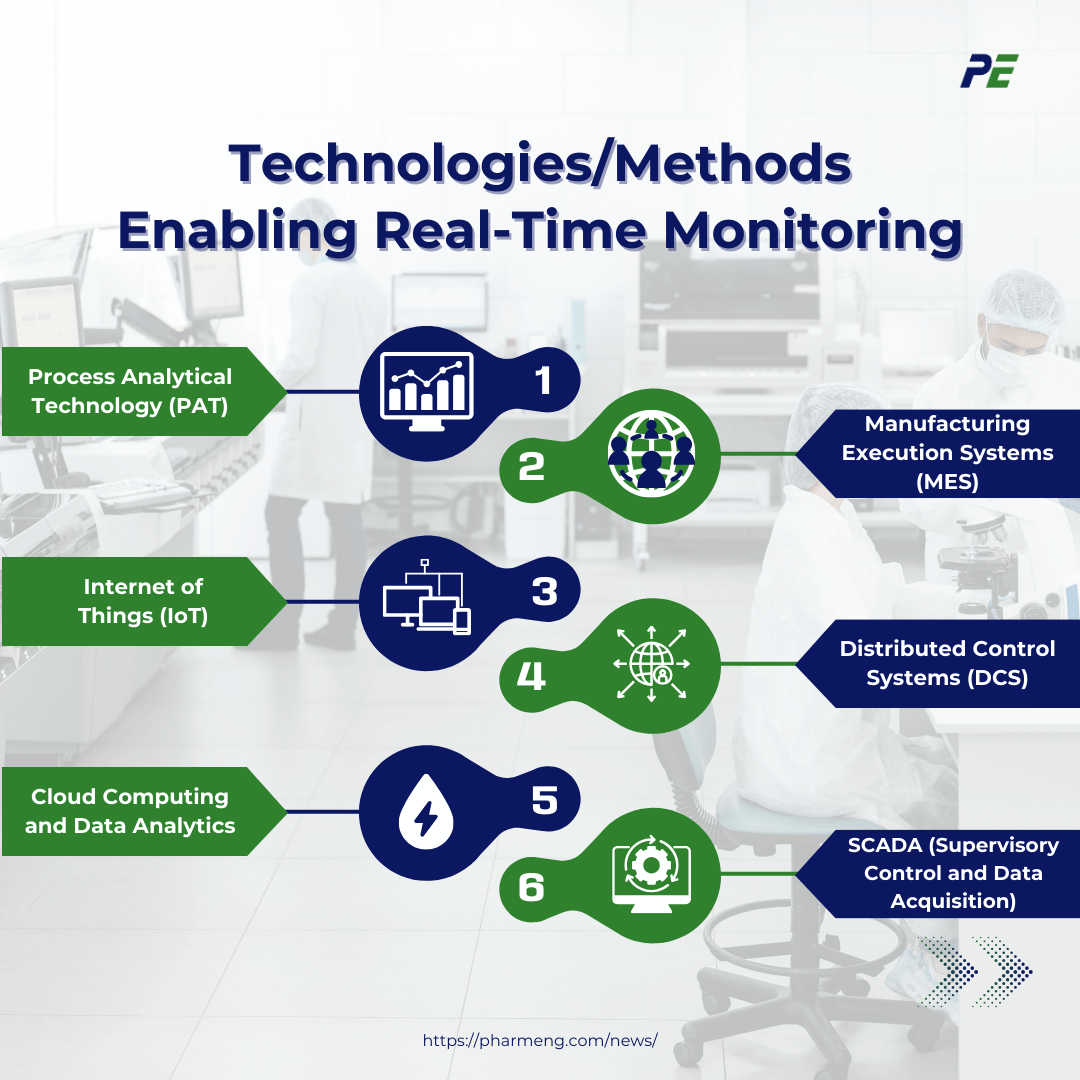
Technologies/Methods Enabling Real-Time Monitoring
Real-time CQV relies on cutting-edge technologies and methodologies that facilitate continuous monitoring and provide immediate feedback during pharmaceutical manufacturing processes. Below are some technologies and methods enabling real-time monitoring in CQV.
1. Process Analytical Technology (PAT)
Overview: PAT is a framework developed by the FDA as part of the Quality by Design (QbD) initiative, designed to analyze and control pharmaceutical manufacturing processes through the measurement of Critical Quality Attributes (CQAs) and Critical Process Parameters (CPPs) in real-time.
Key Features: Near-infrared (NIR), Fourier-Transform Infrared Spectroscopy (FTIR), Raman, Chromatography, Process Control Systems
Benefits:
- Immediate feedback and adjustments during production
- Reduced reliance on end-product testing
- Enhanced understanding of process dynamics
2. Manufacturing Execution Systems (MES)
Overview: MES is a real-time system that controls, monitors, and records the entire manufacturing process. It acts as a bridge between the shop floor and enterprise-level systems, ensuring data integration and seamless process management.
Key Features: Data Collection, Real-Time Visualization, Traceability
Benefits:
- Centralized data management and analysis.
- Enhanced decision-making through real-time insights.
- Improved compliance with regulatory requirements.
3. Internet of Things (IoT)
Overview: IoT involves the network of physical devices embedded with sensors, software, and connectivity, enabling them to collect and exchange data in real-time.
Key Features: Sensor Networks, Connectivity, Predictive Maintenance
Benefits:
- Real-time monitoring of process and equipment conditions.
- Enhanced operational efficiency through predictive analytics.
- Improved process control and quality assurance.
4. Distributed Control Systems (DCS)
Overview: DCS are specialized control systems used to manage complex, large-scale industrial processes. They distribute control functions throughout a process, allowing for real-time adjustments and monitoring.
Key Features: Decentralized Control, Integration with PAT, Advanced Process Control (APC)
Benefits:
- Enhanced process stability and product consistency.
- Real-time process optimization and control.
- Reduced variability and risk of non-compliance.
5. Cloud Computing and Data Analytics
Overview: Cloud computing provides scalable storage and processing power for large datasets generated by real-time monitoring systems. Data analytics tools, including Machine Learning (ML) and Artificial Intelligence (AI), process and analyze this data to extract actionable insights.
Key Features: Scalable Data Storage, Real-Time Data Processing, Machine Learning and AI
Benefits:
- Enhanced data accessibility and collaboration across multiple locations.
- Improved decision-making through real-time insights and predictive analytics.
- Ensures compliance with data integrity standards such as 21 CFR Part 11.
6. SCADA (Supervisory Control and Data Acquisition)
Overview: SCADA utilizes computers, networked data communications, and graphical user interfaces for high-level supervision and management of industrial processes.
Key Features: Data Acquisition, Real-Time Monitoring, Data Logging, Remote Access.
Benefits:
- Minimizes manual interventions and maximizes resource utilization.
- Real-time data visibility helps operators make informed decisions quickly.
- Enables fast identification of deviations or potential failures.
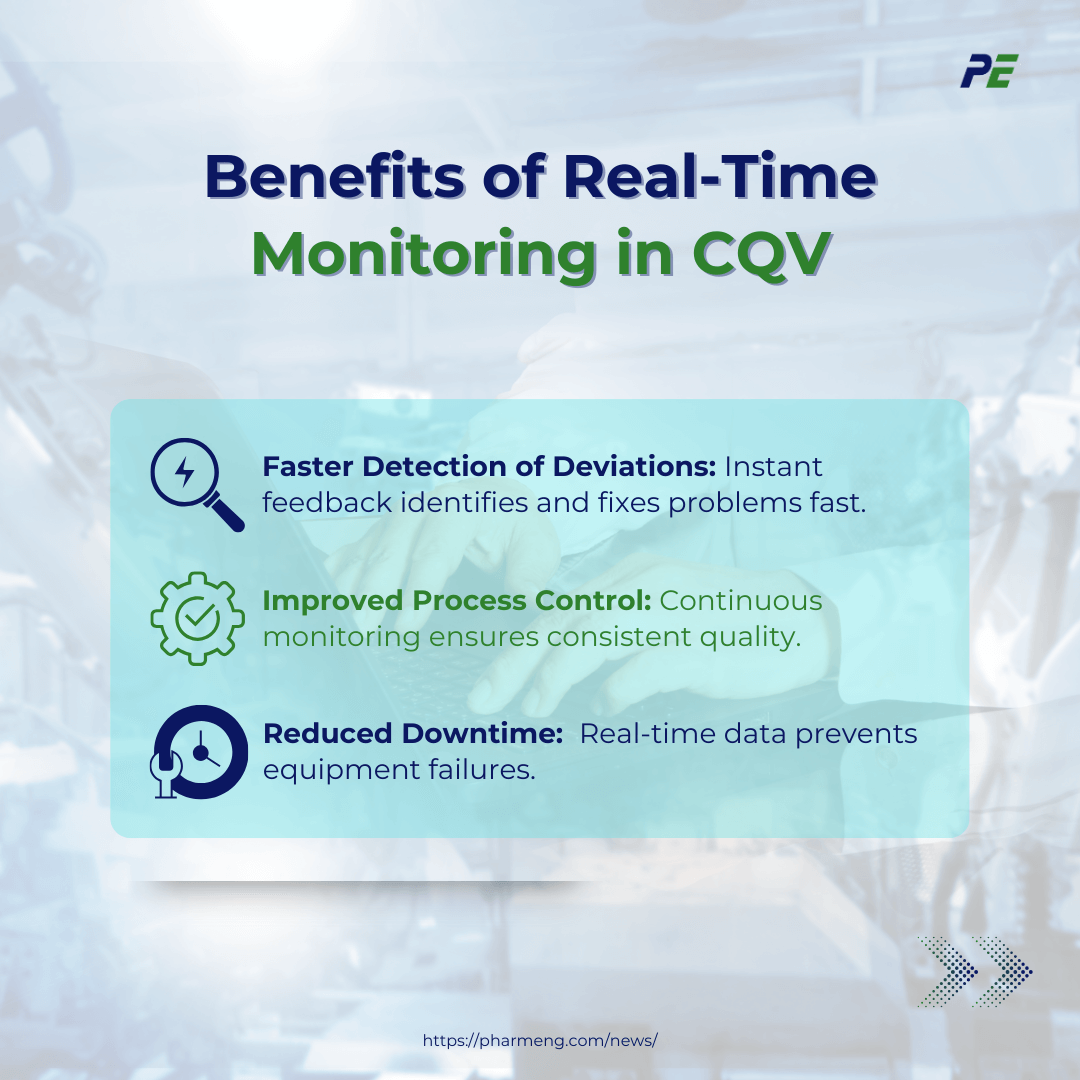
Benefits of Real-Time Monitoring in CQV
The integration of real-time monitoring in CQV offers several key benefits that enhance the efficiency, reliability, and compliance of pharmaceutical manufacturing processes. These include:
- Faster Detection of Deviations. Instant feedback allows for the rapid identification and correction of issues before they escalate, minimizing product rejections.
- Improved Process Control. Continuous tracking of CQAs and CPPs ensures that processes stay within control and thus, maintain product quality throughout production.
- Reduced Downtime. Real-time data from equipment and processes enables predictive maintenance, identifying potential issues before they cause equipment failure. This reduces unplanned downtime and extends equipment life.
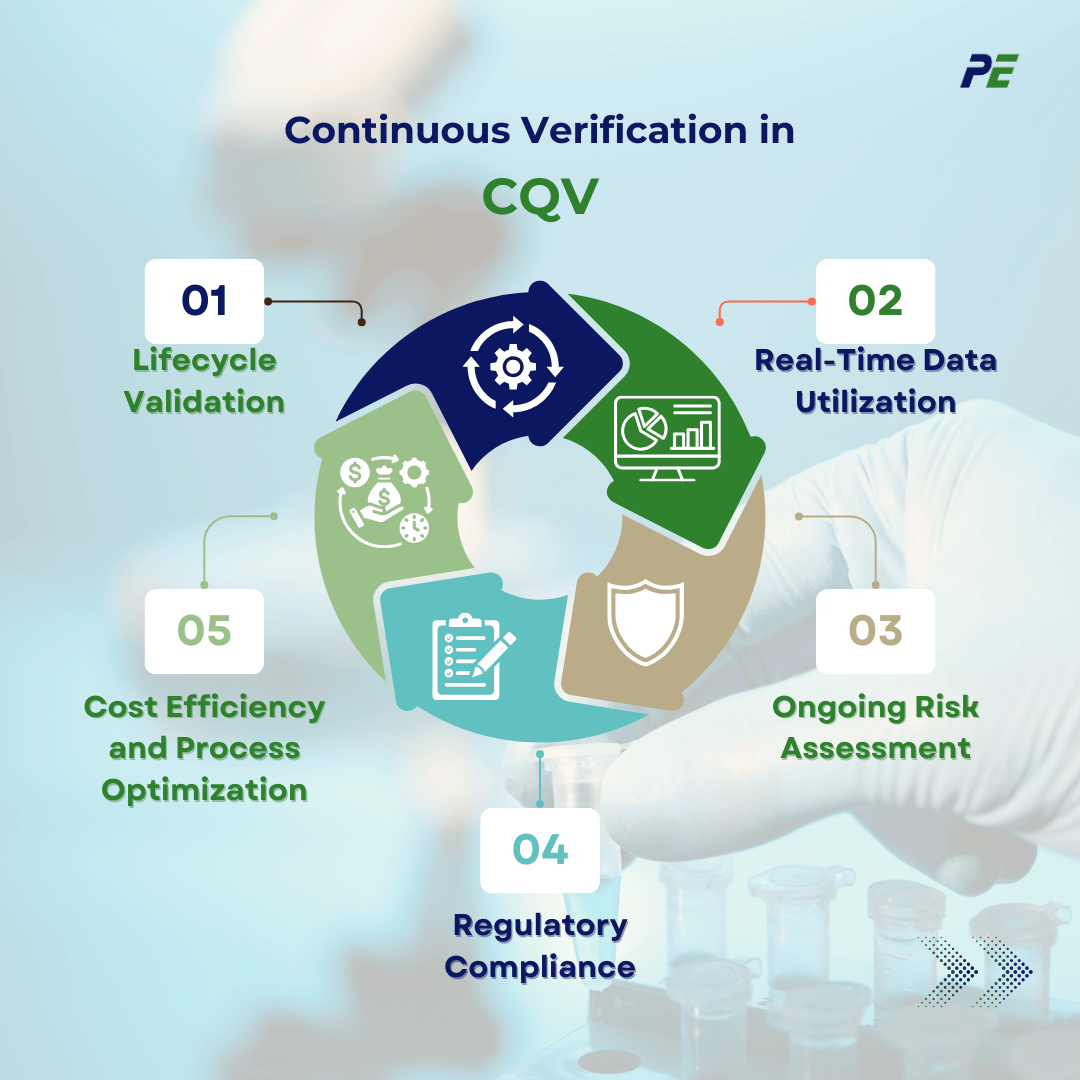
Continuous Verification in CQV
Continuous Verification is a modern approach in pharmaceutical manufacturing that extends beyond traditional, one-time validation events. This method aligns with ICH Q12 (Lifecycle Management) guidelines, ensuring that processes and systems remain in a validated state over time. Key concepts of Continuous Verification include:
1. Lifecycle Validation
Continuous Verification extends beyond initial qualification phases like IQ, OQ, and PQ. It involves real-time monitoring and reassessment throughout the entire lifecycle of the system or process.
2. Real-Time Data Utilization
Continuous Verification relies heavily on real-time data from process monitoring systems. Sensors and automated data capture tools provide ongoing insights into process parameters, ensuring they remain within the defined control limits.
3. Ongoing Risk Assessment
Continuous Verification involves continuous risk assessment and mitigation. As new data is collected, it is used to reassess the risks associated with the process, allowing for dynamic adjustments to control strategies to prevent deviations.
4. Regulatory Compliance
Regulatory bodies such as the FDA and EMA emphasize the importance of maintaining validated states throughout the entire lifecycle of a product or system.
5. Cost Efficiency and Process Optimization
Continuous verification reduces the need for extensive re-validation efforts after changes or updates. This reduces downtime, enhances operational efficiency, and lowers associated costs.
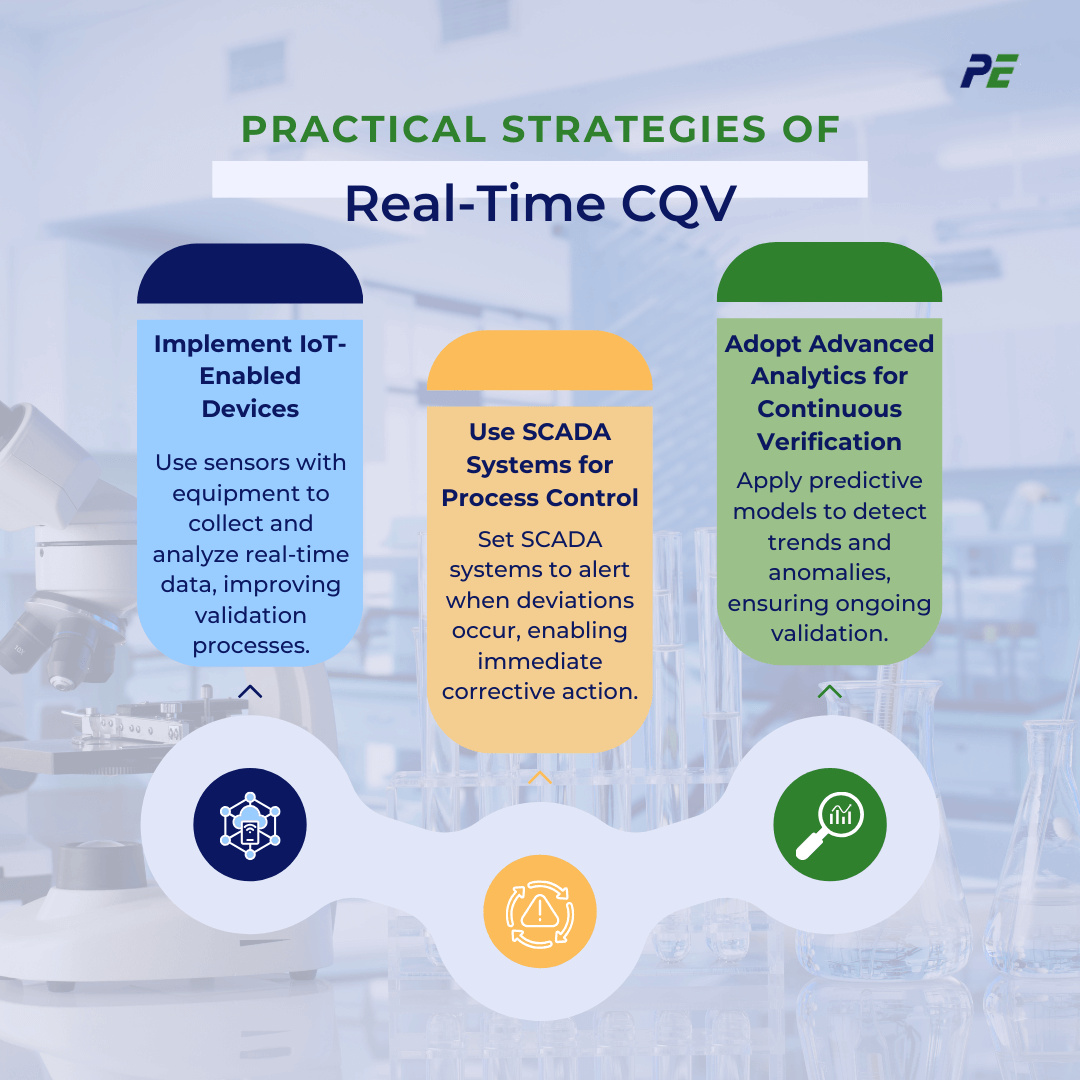
Practical Strategies of Real-Time CQV
To effectively implement Real-Time CQV, practical strategies can be employed to ensure continuous monitoring, faster decision-making, and enhanced process control. Below are practical strategies for adopting real-time CQV:
1. Implement IoT-Enabled Devices
- Practical Approach: Integrate sensors with existing equipment and connect them to a centralized data platform to capture and analyze data continuously for better validation processes.
2. Use SCADA Systems for Process Control
- Practical Approach: Configure SCADA systems to trigger alarms when deviations from predefined parameters are detected, allowing immediate corrective action to ensure validation compliance.
3. Adopt Advanced Analytics for Continuous Verification
- Practical Approach: Implement predictive models that identify trends and anomalies, ensuring systems remain validated throughout their lifecycle.
Conclusion
Real-time CQV and Continuous Verification are transforming pharmaceutical manufacturing by shifting validation from static to dynamic, ongoing processes. Real-time monitoring provides immediate insights into system performance, while Continuous Verification ensures that processes remain in control throughout the entire lifecycle. These approaches ensure consistent product quality, regulatory compliance, and process efficiency. Moving forward, technologies like AI and machine learning will continue to refine real-time CQV systems, enabling even more precise control and optimization in a data-driven industry.
Partnering with PharmEng Technology
Unlock the full potential of your pharmaceutical manufacturing processes with our advanced Real-Time CQV solutions. At PharmEng Technology, we combine deep industry expertise with innovative technologies to ensure your operations remain at the forefront of efficiency and compliance. Our solutions are designed to enhance process monitoring, analytics, and control, providing you with immediate feedback and actionable insights to optimize your manufacturing processes.
Ready to transform your operations? Visit our website at www.pharmeng.com for more details or contact us directly at info.asia@pharmeng.com. Let’s collaborate to elevate your manufacturing excellence with real-time precision and compliance

About PharmEng Technology
PharmEng Technology is a global consulting firm specializing in pharmaceutical engineering, regulatory affairs, and compliance. With a commitment to quality and innovation, PharmEng Technology provides comprehensive solutions to meet the evolving needs of the pharmaceutical and biotechnology industries.
Contact Information
PharmEng Technology
Email: info.asia@pharmeng.com





