Ensuring Data Integrity: The Pillar of Successful CQV Part 2
by Dhika Prameswari and Rida Hadirah Ramli
In the previous article, the fundamental aspects of data integrity were discussed, including its importance, the challenges in maintaining it, and the best practices for ensuring data integrity within the CQV processes. Building on that foundation, this article delves deeper into the crucial roles of data integrity, regulatory frameworks, and data management in the CQV.
Role of Technology in Data Integrity
Modern technology has significantly transformed how data integrity is maintained in the CQV processes. Tools like digital signatures and automated data capture systems play a crucial role in enhancing data integrity by minimizing human error and ensuring comprehensive data traceability.
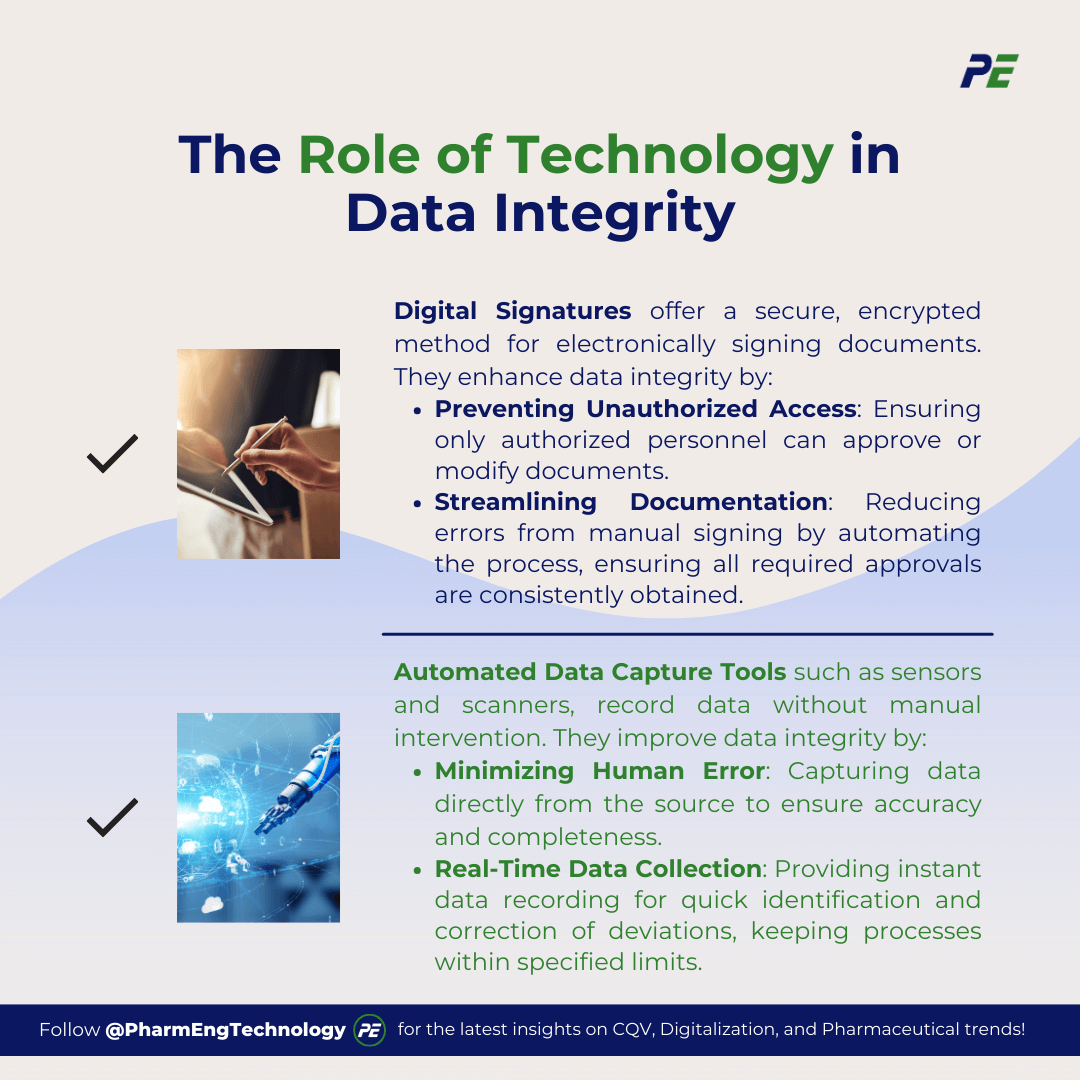
1. Digital Signatures
Digital signatures offer a secure and verifiable way to sign documents electronically. Unlike traditional handwritten signatures, they are encrypted and tied to a specific individual, ensuring the signer is authenticated. This technology enhances data integrity by:
- Preventing Unauthorized Access: Digital signatures ensure that only authorized personnel can approve or modify documents, reducing the risk of unauthorized changes.
- Streamlining Documentation: Automating the signing process eliminates the errors associated with manual signing, such as missing or incorrect signatures, ensuring that all required approvals are consistently obtained.
2. Automated Data Capture Tools
Automated data capture tools, such as sensors, scanners, and software systems, automatically collect and record data without manual intervention. These tools enhance data integrity in several ways:
- Minimizing Human Error: By capturing data directly from the source, these tools ensure that the recorded information is accurate and complete.
- Real-Time Data Collection: Automated systems can capture data in real time. This allows for quick identification and correction of deviations, ensuring the processes are within the specified limits.
Regulatory Requirements for Data Integrity
The regulatory landscape for data integrity in CQV is shaped by stringent guidelines and standards set by global regulatory bodies like the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the World Health Organization (WHO). These regulations are designed to ensure that data used in CQV processes are accurate, reliable, and compliant with industry standards, ultimately safeguarding product quality and patient safety.
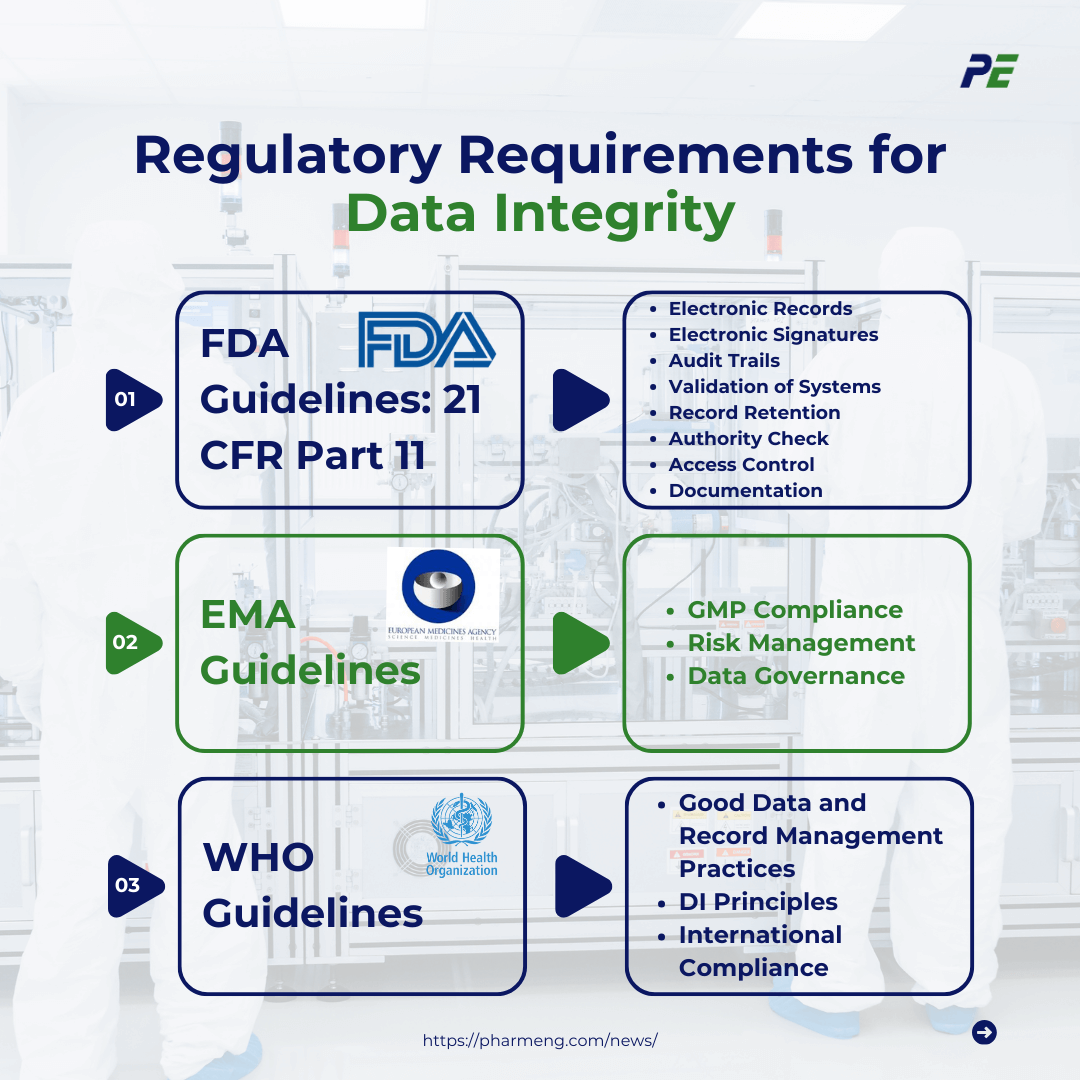
1. FDA Guidelines: 21 CFR Part 11: Critical regulation for the management of electronic records and signatures in the pharmaceutical, biotechnology, and medical device industries. Key aspects include:
- Electronic Records: Part 11 establishes requirements for the creation, modification, maintenance, and storage of electronic records.
- Electronic Signatures: The regulation requires that electronic signatures be uniquely identifiable to the individual signing.
- Audit Trails: Part 11 necessitates the implementation of audit trails that capture all changes made to electronic records, including who made the change, when it was made, and what was changed.
- Validation of Systems: Systems that handle electronic records and signatures must be validated to ensure they function.
- Record Retention: The system must ensure that records are easily retrievable and preserved in a format that is accessible and readable for the entire retention period.
- Authority Check: The system must ensure that only authorized individuals have access to the system and perform electronic signatures on records.
- Access Control: Implement security measures to ensure that only authorized personnel have access to the system.
- Documentation: Proper documentation must be maintained to demonstrate compliance with Part 11 requirements. This includes validation protocols, standard operating procedures (SOPs), user manuals, and training records.
2. EMA Guidelines: Provide comprehensive guidelines that emphasize the importance of data integrity in ensuring the safety, efficacy, and quality of pharmaceutical products. The EMA’s guidelines align with those of the FDA but also focus on:
- GMP Compliance: This includes ensuring that data is accurate, complete, and maintained in a manner that allows for accurate reconstruction of activities. EMA’s guidelines tie into the broader GMP framework, which is essential for ensuring data integrity at every stage of production.
- Risk Management: EMA encourages a risk-based approach to data integrity, based on the ICH Q9 Quality Risk Management guidelines. Organizations are advised to assess potential risks to data throughout its lifecycle, identifying critical control points where data integrity may be compromised.
- Data Governance: EMA guidelines advocate for robust data governance frameworks that define roles, responsibilities, and procedures for managing data integrity. This includes training personnel and ensuring that data management systems are designed to meet regulatory requirements.
3. WHO Guidelines: WHO guidelines on data integrity reflect a global perspective, offering recommendations that align with both FDA and EMA regulations while also considering the needs of different regions:
- Good Data and Record Management Practices: WHO guidelines emphasize the importance of managing electronic and paper records. This includes ensuring data is complete, consistent, and accurate from its creation through its lifecycle.
- DI Principles: WHO guidelines support the ALCOA+ principles to guide organizations in maintaining data integrity in all aspects of CQV. These principles are not only limited to WHO guidelines but are globally recognized and endorsed by other regulatory bodies like the FDA and EMA as part of the foundational elements of data integrity.
- International Compliance: WHO guidelines are designed to be adaptable across different regulatory environments, ensuring that organizations can maintain data integrity while complying with various international standards.
Compliance with regulations like 21 CFR Part 11 and guidelines from the EMA, ICH, and WHO is critical for several reasons:
- Regulatory Approval: Adhering to data integrity guidelines is essential for obtaining and maintaining regulatory approval for pharmaceutical products.
- Product Quality and Safety: Ensuring data integrity is crucial for verifying that products meet their intended quality specifications and are safe for patient use.
- Reputation and Trust: Compliance with data integrity regulations builds trust with regulatory authorities, customers, and stakeholders. It demonstrates an organization’s commitment to maintaining high standards of quality and safety.
Data Management in CQV
Data management practices are crucial for maintaining data integrity by providing structured processes and systems for securely collecting, storing, and analyzing data. Below is the importance of effective data management in CQV:
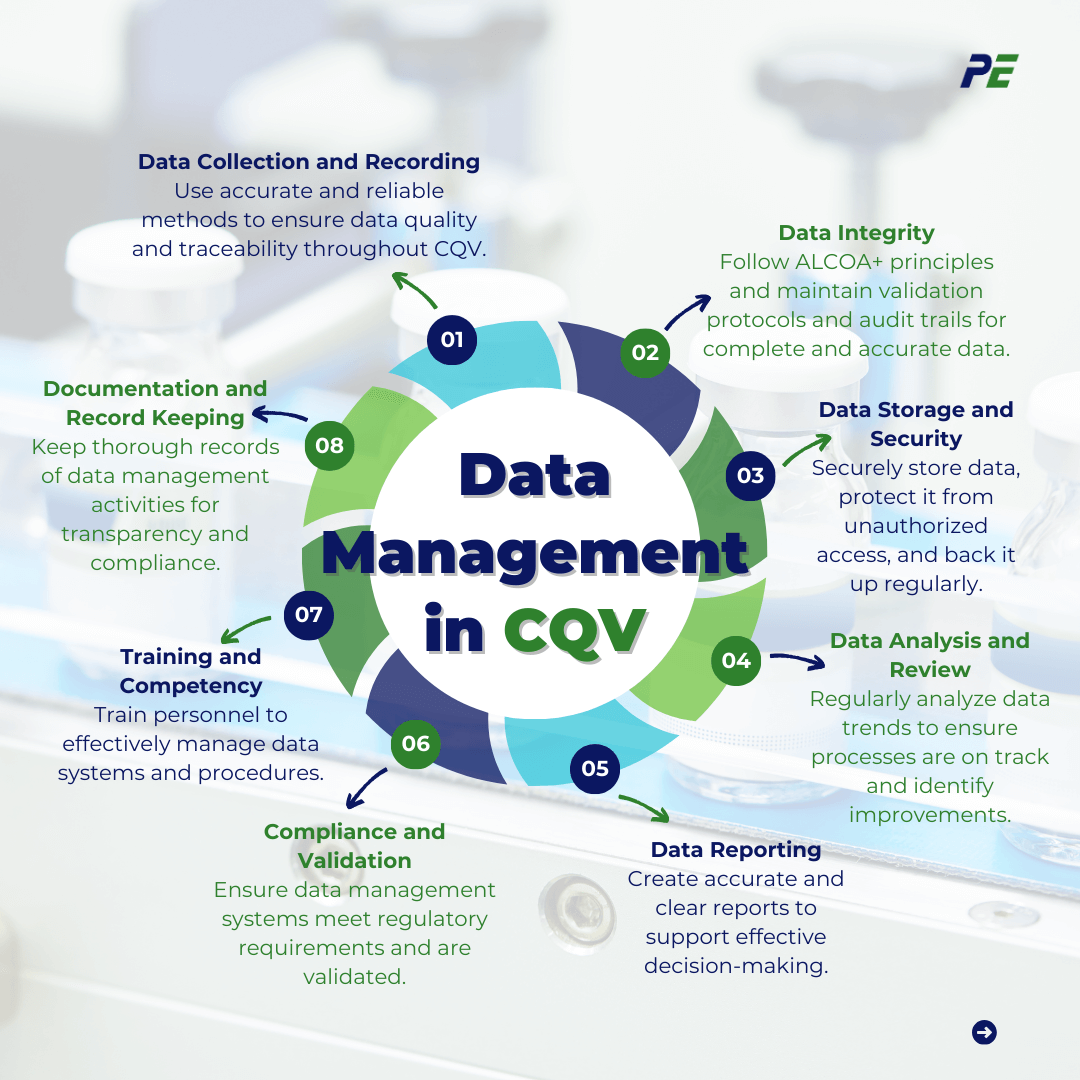
- Data Collection and Recording: Ensure that data collection methods are accurate, consistent, and reliable across all stages of CQV to ensure data quality and traceability.
- Data Integrity: Adhere to the ALCOA+ principles, ensure that data validation protocols are in place, and audit trails are maintained to track all changes to data. These principles, recognized globally, are key to ensuring data is complete, accurate, and trustworthy.
- Data Storage and Security: Data must be stored in secure environments, and protected from unauthorized access, and regular backups should be conducted to ensure that data is preserved and recoverable.
- Data Analysis and Review: Implement statistical tools to analyze data for trends. Regular reviews of collected data are necessary to ensure that processes remain within validated parameters and to identify any deviations or areas for improvement.
- Data Reporting: Reports generated from CQV activities should be accurate, comprehensive, and easy to interpret, facilitating effective decision-making.
- Compliance and Validation: Data management systems must comply with regulatory requirements such as 21 CFR Part 11. All systems used for data collection, storage, and analysis must be validated to ensure data integrity and reliability.
- Training and Competency: All personnel involved in data management activities should receive proper training on the systems and procedures they will use, to ensure competency in handling data.
- Documentation and Record Keeping: Maintain thorough documentation of all data management activities, including data collection methods, validation protocols, training records, and audit trails. Proper documentation supports transparency, traceability, and regulatory compliance.
Conclusion
By highlighting the role of modern technologies, the regulatory landscape, and best practices in data management, it underscores the necessity of maintaining rigorous standards to ensure product quality and patient safety. The emphasis on compliance with regulations such as 21 CFR Part 11, alongside secure and efficient data management, reinforces the critical nature of these practices in safeguarding the integrity of CQV processes. This conclusion ties together the various elements that contribute to robust and reliable validation activities, which are essential in the pharmaceutical and biotech industries.
Partnering with PharmEng Technology
Partnering with PharmEng Technology means ensuring the highest level of data integrity throughout your CQV processes. We leverage advanced technology like digital signatures and automated data capture systems to minimize errors, improve traceability, and comply with stringent regulatory standards like FDA 21 CFR Part 11. Our team is well-versed in global guidelines from the EMA and WHO, ensuring your data remains secure, accurate, and reliable. With our expertise, you can confidently safeguard product quality and patient safety.
At PharmEng Technology, we understand that compliance and data management are critical to successful CQV. Our tailored solutions are designed to help your organization meet regulatory requirements while enhancing efficiency. Whether you’re looking to streamline your data collection or ensure proper validation, we’re here to provide end-to-end support. Ready to elevate your data integrity practices? Contact us via email at info.asia@pharmeng.com to learn more!

About PharmEng Technology
PharmEng Technology is a global consulting firm specializing in pharmaceutical engineering, regulatory affairs, and compliance. With a commitment to quality and innovation, PharmEng Technology provides comprehensive solutions to meet the evolving needs of the pharmaceutical and biotechnology industries.
Contact Information
PharmEng Technology
Email: info.asia@pharmeng.com





