Ensuring Data Integrity: The Pillar of Successful CQV Part 1
by Dhika Prameswari and Rida Hadirah Ramli
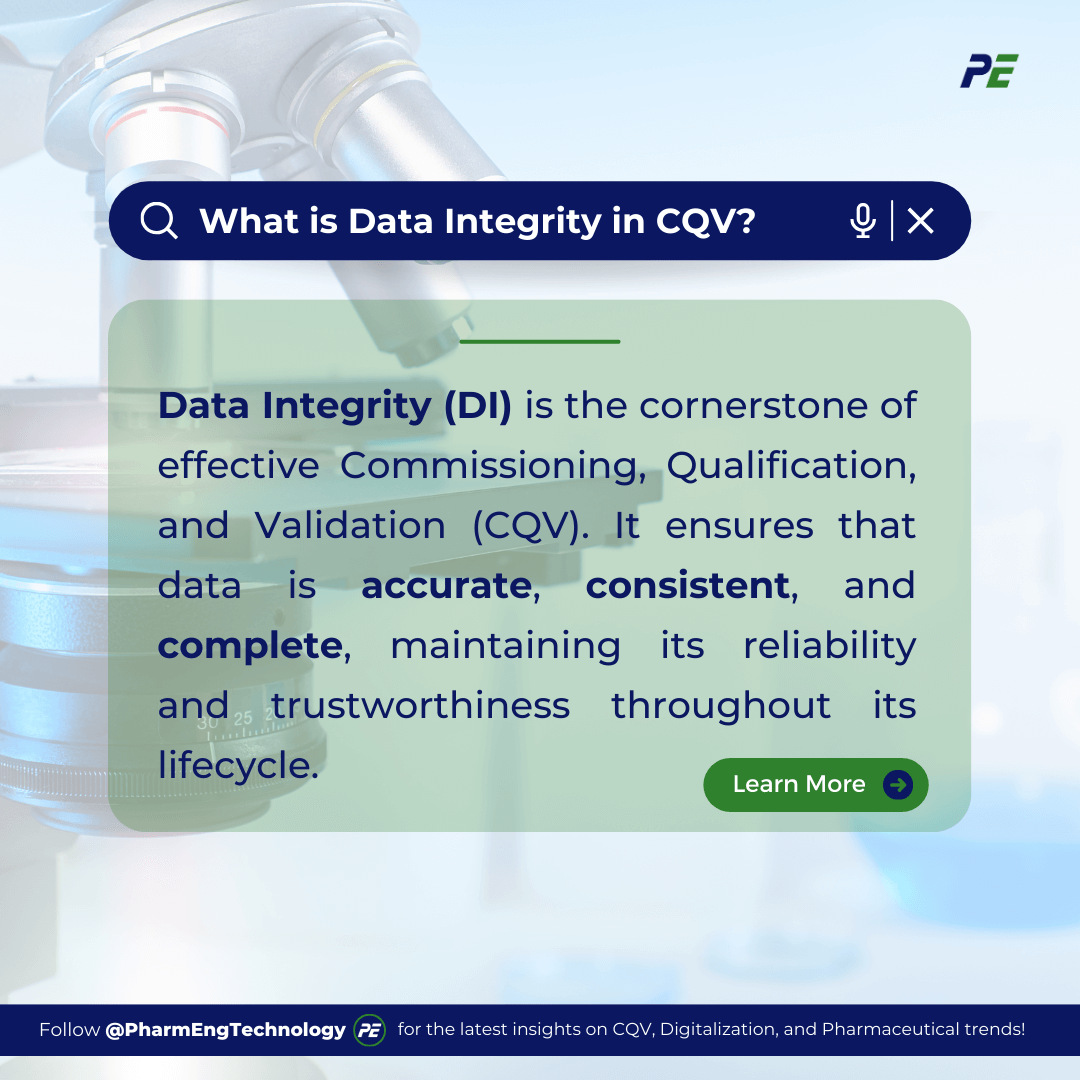
Data Integrity (DI) refers to the accuracy, consistency, and completeness of data throughout its lifecycle. In the context of the pharmaceutical industry, it is the assurance that data is recorded, stored, and maintained in a way that is true to the original information and remains unchanged unless properly authorized and documented. This concept is fundamental to maintaining trust in the data used for decision-making, product quality and safety, and achieving compliance with regulatory requirements. This article provides a deep dive into the importance of DI, the challenges of maintaining it, and best practices for ensuring DI in Commissioning, Qualification, and Validation (CQV).
Understanding Data Integrity
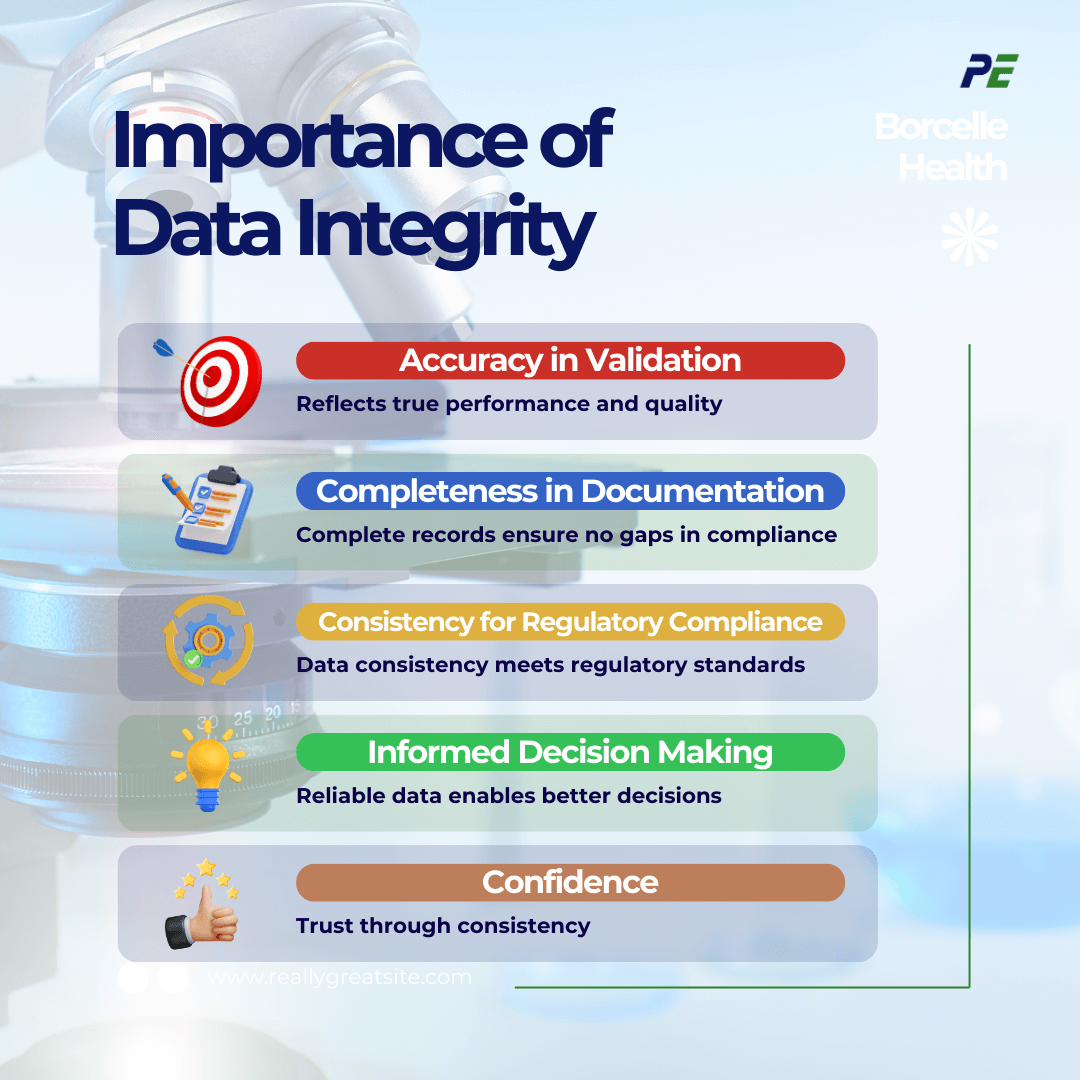
Data Integrity is crucial for making informed decisions, therefore, the importance of DI in CQV are:
- Accuracy in Validation
- DI ensures that the data collected during validation is accurate, reflecting the true performance of equipment, systems, and processes. Inaccurate data can lead to flawed validation, potential production failures, and compromised product quality.
- Completeness in Documentation
- Complete data is crucial for maintaining a thorough record of all validation activities, including test results, deviations, and corrective actions. Incomplete data can lead to gaps in documentation, making it difficult to demonstrate compliance with regulatory standards.
- Consistency for Regulatory Compliance
- FDA and EMA require that data used in the validation of pharmaceutical processes is consistent and trustworthy. Consistency in data ensures that processes are reproducible and the products meet predefined quality standards.
- Informed Decision Making
- Consistent and accurate data enables informed decision-making regarding process improvements, risk assessments, and the implementation of corrective and preventive actions (CAPA).
- Confidence
- Maintaining DI fosters trust with regulators, customers, and internal stakeholders, demonstrating the company’s dedication to producing safe, effective, and high-quality products.
Challenges in Maintaining Data Integrity
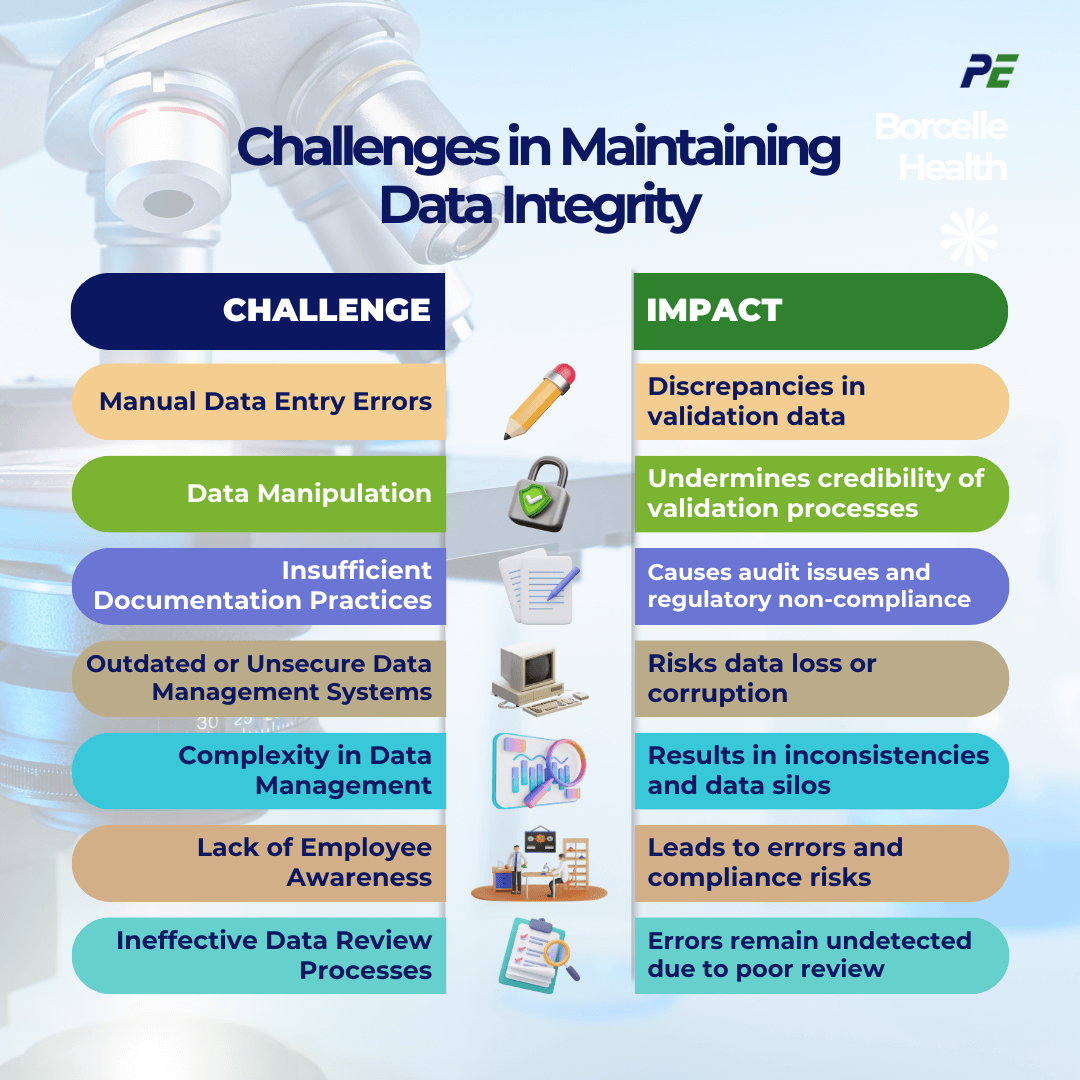
Maintaining DI during CQV is critical to ensure that pharmaceutical processes and products meet stringent regulatory standards. However, various challenges can affect data integrity, leading to significant operational and compliance issues. The following table outlines common challenges and their associated impacts:
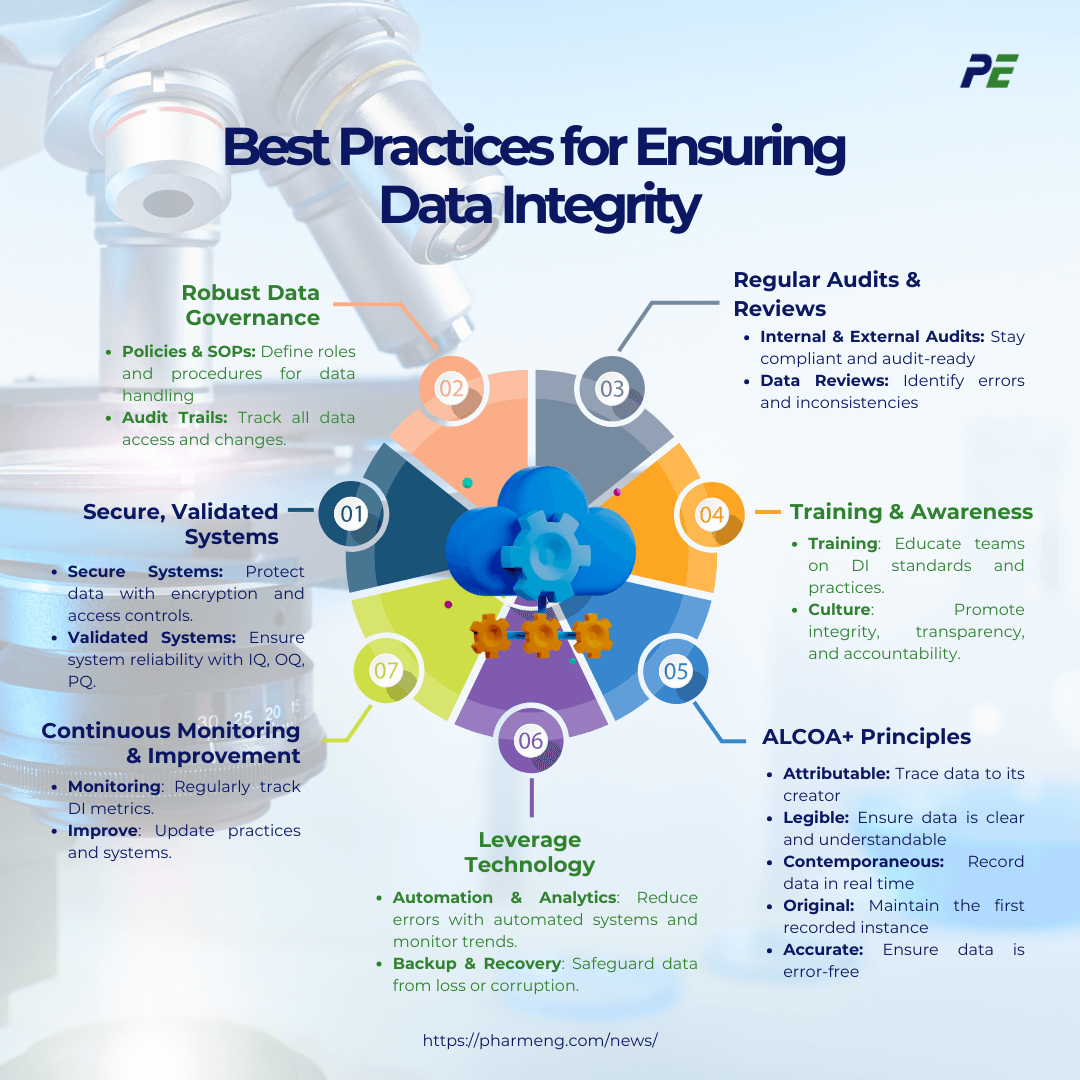
Best Practices for Ensuring Data Integrity
Ensuring DI in Commissioning, Qualification, and Validation (CQV) is essential for maintaining compliance, producing high-quality products, and upholding the credibility of the pharmaceutical industry. Below is an outline of best practices to ensure DI in CQV:
- Use of Secure, Validated Systems
- Secure Systems: Ensure that all data management systems used in CQV are secure, with appropriate access controls, encryption, and audit trails. This prevents unauthorized access, data breaches, and tampering.
- Validated Systems: Validate all computerized systems used in CQV to confirm that they perform as intended. This includes performing Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) to ensure system reliability.
- Implement Robust Data Governance Frameworks
- Data Governance Policies: Develop and enforce comprehensive data governance policies that define data handling, storage, and management procedures. This includes establishing roles and responsibilities for data stewardship.
- Standard Operating Procedures (SOPs): Implement SOPs that cover all aspects of data management, including data collection, entry, storage, processing, and archiving. Ensure that these procedures are well-documented and regularly updated.
- Audit Trails: Maintain detailed audit trails that record all data-related activities, including who accessed or modified the data, and when these actions occurred. This helps trace any issues back to their source.
- Regular Audits and Reviews
- Internal Audits: Conduct regular internal audits to assess compliance with DI standards and identify potential risks. These audits should review data management practices, system validations, and adherence to SOPs.
- External Audits: Be prepared for external audits by regulatory bodies. Regular internal reviews can help ensure that all systems and practices are audit-ready at all times.
- Data Review Processes: Implement thorough and systematic data review processes to identify and rectify any inconsistencies or errors. This includes peer reviews and management oversight.
- Training and Personnel Awareness
- Training Programs: Provide regular training to all personnel involved in CQV on the importance of DI, the specific requirements for maintaining it, and the consequences of non-compliance.
- Continuous Learning: Encourage continuous learning and development to keep personnel updated on the latest DI practices, regulatory requirements, and technological advancements.
- Culture of Integrity: Foster a culture that prioritizes DI. This can be achieved by promoting transparency, accountability, and ethical behaviour in data handling.
- Adhere to the ALCOA+ Principles
- ALCOA+ Principles: Ensure that all data meets the ALCOA+ principles, which are foundational to maintaining DI. These principles include:
- Attributable: Data should be traceable to the person who generated or modified it, with clear records of who did what and when.
- Legible: Data should be clear and understandable. It should be recorded in a way that allows anyone reviewing it to comprehend its meaning without ambiguity.
- Contemporaneous: Data should be recorded at the time the activity is performed, not retrospectively. This ensures accuracy and relevance.
- Original: The first recorded instance of data should be preserved. This could be in paper or electronic format but must be the original source of truth.
- Accurate: Data must be correct, free from errors, and truly represent the activity or event being recorded.
- Complete: All relevant data, including any changes or amendments, should be captured. Nothing should be omitted that might alter the context or understanding.
- Consistent: Data should be consistent across different systems and over time. Consistent data ensures that results are reproducible and reliable.
- Enduring: Data should be stored in a manner that preserves its integrity over the required retention period, ensuring it remains accessible and unaltered.
- Available: Data should be readily accessible for review, audits, and inspections throughout its lifecycle.
- ALCOA+ Principles: Ensure that all data meets the ALCOA+ principles, which are foundational to maintaining DI. These principles include:
- Leverage Technology for Data Integrity
- Automated Data Capture: Use automated data capture systems where possible to reduce the risk of human error and ensure data accuracy.
- Data Analytics: Implement advanced data analytics tools to monitor data trends, identify anomalies, and ensure that data remains within expected parameters.
- Backup and Recovery: Establish robust data backup and recovery procedures to protect against data loss or corruption.
- Regular Monitoring and Continuous Improvement
- Continuous Monitoring: Regularly monitor DI metrics and implement corrective actions when issues are identified. This includes tracking changes, reviewing DI logs, and responding to any deviations promptly.
- Continuous Improvement: Foster a mindset of continuous improvement in DI practices. This involves regularly reviewing and updating processes, systems, and training programs to adapt to new challenges and technologies.
Conclusion
In summary, data integrity is crucial for the pharmaceutical industry’s CQV processes. It ensures accurate, complete, consistent, and trustworthy data, enabling informed decisions and regulatory compliance. Challenges include manual data entry errors, manipulation, and outdated systems. Best practices include secure systems, robust data governance frameworks, regular audits, personnel training, and adhering to ALCOA+ principles. Adhering to these practices ensures data integrity and product quality.
Partnering with PharmEng Technology
Ensure your pharmaceutical validation processes meet the highest standards of Data Integrity (DI) with PharmEng Technology. Our expertise in automation and optimized data management helps maintain accuracy, consistency, and security throughout your Commissioning, Qualification, and Validation (CQV) processes. We offer tailored solutions that address your specific needs while ensuring regulatory compliance and operational excellence.
Discover how PharmEng Technology can enhance your data integrity practices. Visit us at www.pharmeng.com or contact us at info.asia@pharmeng.com to learn more about our innovative solutions and how we can support your success.

About PharmEng Technology
PharmEng Technology is a global consulting firm specializing in pharmaceutical engineering, regulatory affairs, and compliance. With a commitment to quality and innovation, PharmEng Technology provides comprehensive solutions to meet the evolving needs of the pharmaceutical and biotechnology industries.
Contact Information
PharmEng Technology
Email: info.asia@pharmeng.com





