
Risk-Based CQV: Enhancing Efficiency and Compliance Part 1
by Dhika Prameswari and Rida Hadirah Ramli
In the pharmaceutical and biotechnology industries, the shift toward a risk-based approach in CQV has become increasingly critical. This approach emphasizes focusing efforts on high-risk areas, thereby improving efficiency and ensuring compliance with regulatory requirements. By adopting a risk-based methodology, companies can better allocate resources, streamline validation processes, and enhance product quality.
What is a Risk-Based Approach & What are the Principles of Risk Management of CQV?
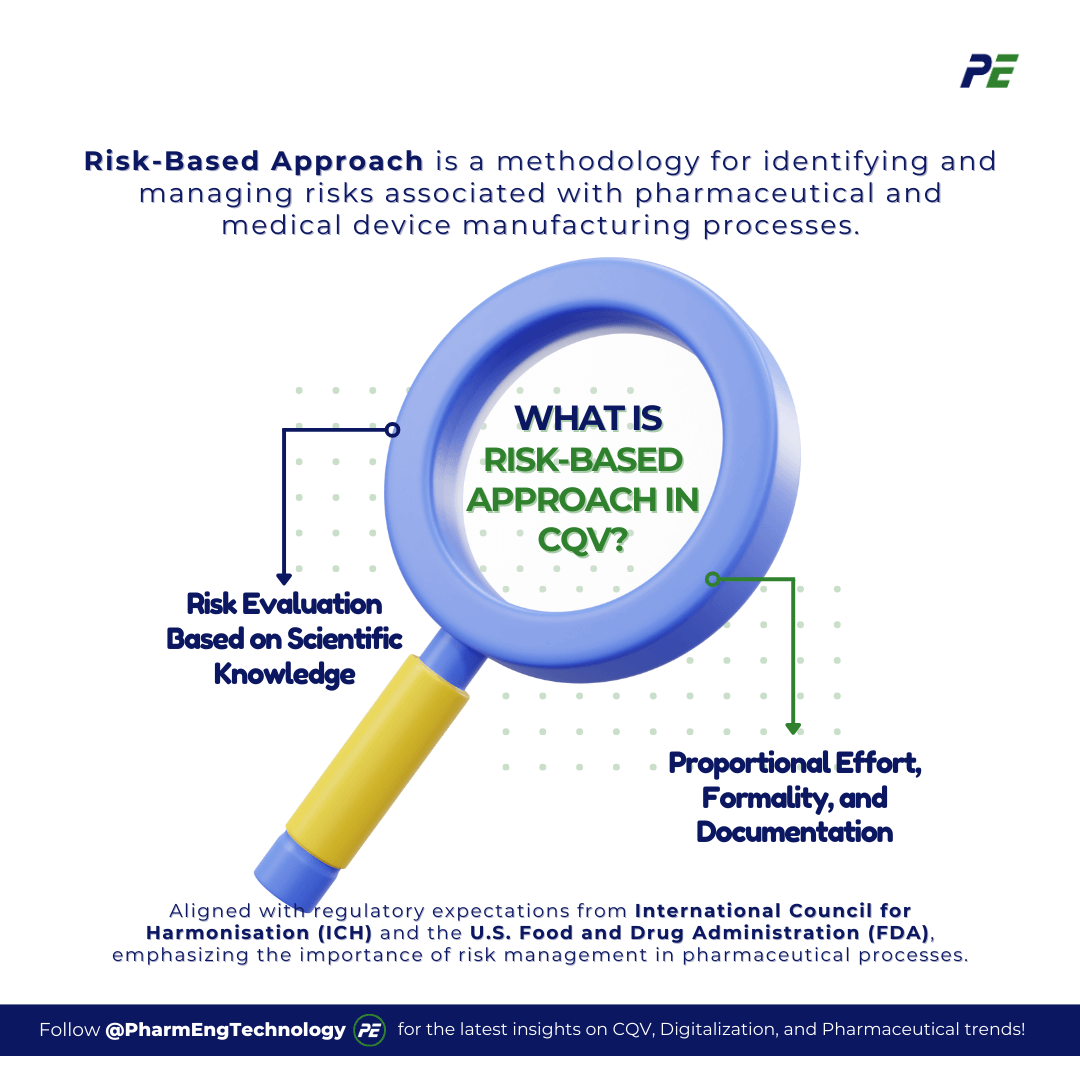
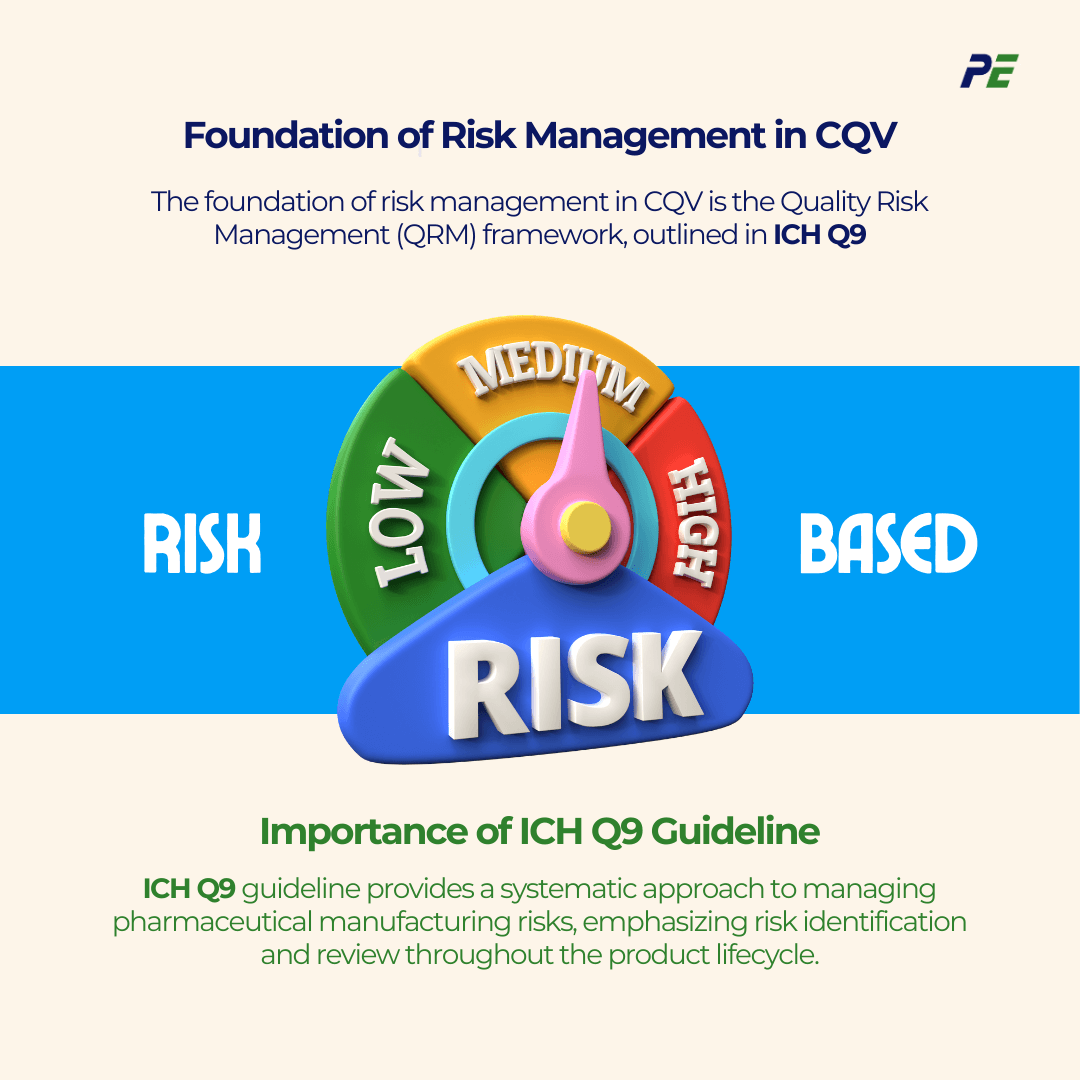
The Risk-Based Approach is a methodology for identifying and managing risks associated with pharmaceutical and medical device manufacturing processes. This approach is aligned with regulatory expectations, such as those from the International Council for Harmonisation (ICH) and the U.S. Food and Drug Administration (FDA), which emphasize the importance of risk management in pharmaceutical processes. The foundation of risk management in CQV is the Quality Risk Management (QRM) framework, which is outlined in ICH Q9. Below are the core principles of QRM:
- Risk Evaluation Based on Scientific Knowledge: The assessment of risk to quality must be grounded in scientific understanding, ensuring that decisions are made with the goal of protecting the patient. This principle emphasizes the importance of a scientific approach in identifying and mitigating risks that could impact product quality and, ultimately, patient safety.
- Proportional Effort, Formality, and Documentation: The effort, formality, and level of documentation required in the QRM process should be proportional to the level of risk involved. This means that more significant risks demand greater attention and resources, while lesser risks can be managed with a more streamlined approach. This principle ensures that resources are allocated effectively, focusing on areas where they are most needed to maintain product quality.
The ICH Q9 guideline on QRM provides a systematic approach to managing pharmaceutical manufacturing risks and quality assurance risks. It emphasizes the importance of integrating risk identification into risk review of the product lifecycle, from development to commercial production.
More details will be provided in the continuation of this article, offering a deeper dive into the practical steps for implementing a risk-based CQV framework and the supporting regulatory guidelines, ensuring all aspects are thoroughly addressed.
Challenges in Traditional CQV Approaches
Traditional Commissioning, Qualification, and Validation (CQV) methods, while foundational in ensuring compliance and quality in pharmaceutical and biotechnology industries, come with several limitations that can hinder efficiency and effectiveness. These limitations include:
- Lack of Customization: Traditional CQV methods often apply the same validation protocols across all systems and processes, regardless of their specific risk profiles or criticality. This uniform approach can lead to unnecessary validation activities in areas that do not require them, while potentially underestimating the needs of more critical systems.
-
- Excessive Focus on Low-Risk Areas: Traditional methods can lead to excessive focus on low-risk areas, resulting in over-validation and over-documentation. This wastes valuable time and resources while diverting attention from higher-risk areas that require more rigorous validation efforts.
- Overuse of Time and Resources: Applying extensive validation protocols across a project, regardless of risk, can lead to resource wastage. Personnel, time, and financial resources may be spent on tasks that have little impact on the product’s overall quality or compliance.
- Complexity and Rigid Structures: Traditional CQV methods are often rigid and can be difficult to adapt to the specific needs of a project. Managing the complexity of validation processes can be challenging especially in large-scale projects, leading to inefficiencies and potential errors.
- Inflexibility in Adapting to Change: As regulatory expectations evolve and new technologies emerge, traditional methods may struggle to keep pace. This inflexibility can result in difficulties in maintaining compliance and managing validation activities effectively, particularly in dynamic or highly regulated environments.
These constraints highlight the importance of more current, adaptable, and risk-based approaches to CQV. Companies may increase the efficiency and efficacy of their CQV processes by prioritizing essential areas, optimizing resource utilization, and adjusting to the unique demands of each project, resulting in higher quality, compliance, and overall project success.

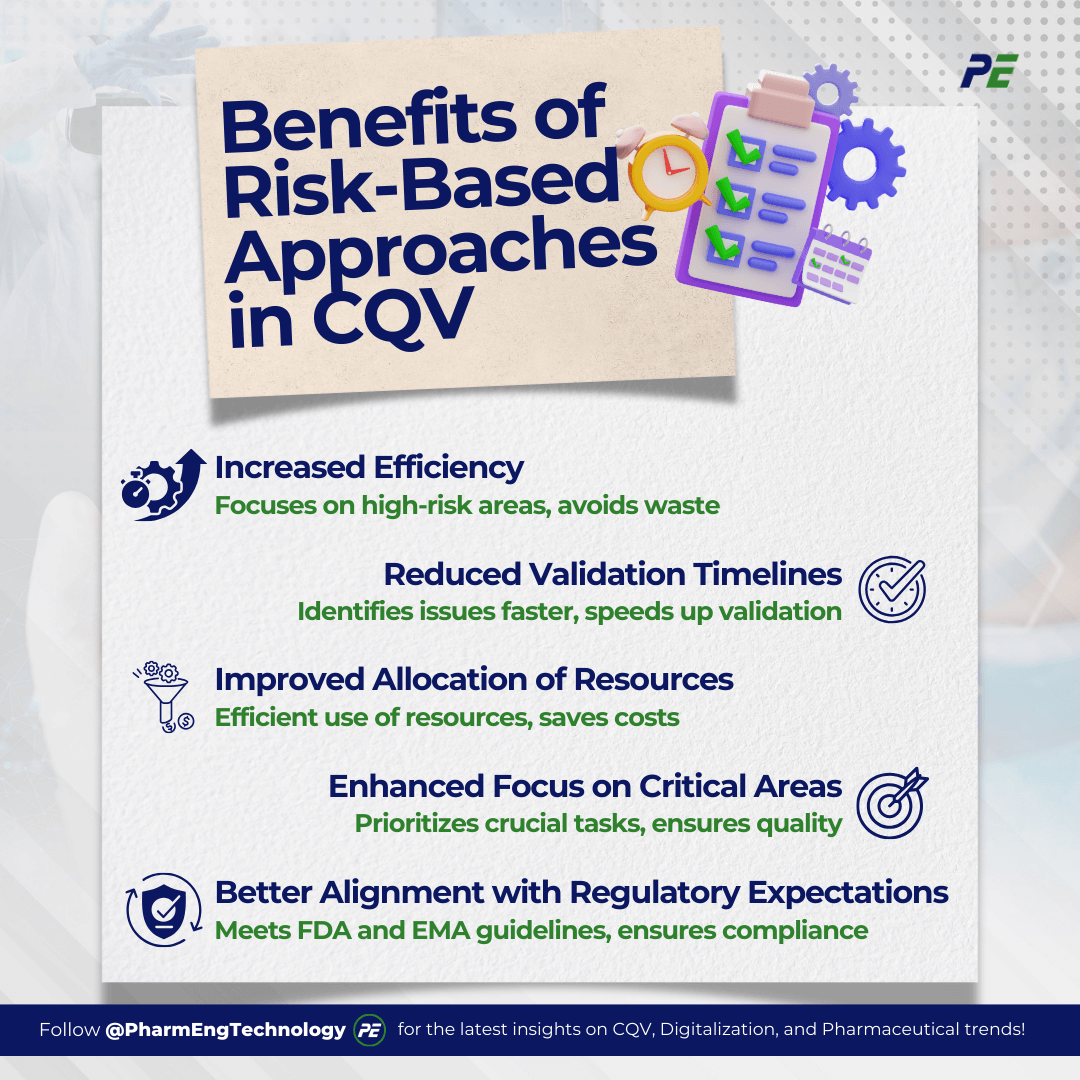
Benefits of Risk-Based Approaches in CQV
Risk-based Commissioning, Qualification, and Validation (CQV) offer several advantages over traditional methods, particularly in terms of efficiency, resource allocation, and regulatory compliance. Here is a breakdown of the key benefits:
- Increased Efficiency, risk-based CQV streamlines the process by focusing on high-risk areas, eliminating redundant or low-value activities, and allowing teams to concentrate on critical tasks impacting product quality and safety.
- Reduced Validation Timelines, risk-based CQV focuses on critical systems, enabling quicker identification and mitigation of potential issues, reducing validation timelines, and providing a competitive edge in the pharmaceutical and biotechnology industries.
- Improved Allocation of Resources, the risk-based approach allocates resources efficiently, focusing on high-risk areas and minimizing low-risk areas, resulting in cost savings in the CQV process.
- Enhanced Focus on Critical Areas, risk-based CQV prioritizes critical systems and processes for thorough validation, enhancing project quality and compliance. It enables early identification and management of potential issues, reducing delays.
- Better Alignment with Regulatory Expectations, Regulatory bodies such as the FDA and EMA endorse risk-based approaches as they encourage transparency and compliance in CQV by thoroughly documenting risk assessments and validation activities.
Conclusion
The pharmaceutical industry is adopting a risk-based approach in Commissioning, Qualification, and Validation (CQV). This method prioritizes high-risk areas, optimizes resource allocation, and aligns with regulatory expectations. This approach increases efficiency, reduces validation timelines, and enhances product quality and patient safety. By integrating risk management principles, pharmaceutical companies can navigate complexities with greater agility and precision, meeting regulatory demands while driving innovation and maintaining high-quality standards.
Why PharmEng Technology?
PharmEng Technology offers a streamlined solution to traditional commissioning and qualification processes, eliminating paper-based documentation inefficiencies. Our platform enhances accuracy and efficiency through electronic execution, ensuring compliance with regulatory requirements. With a centralized repository for easy access, sharing, and tracking of documentation, PharmEng Technology significantly boosts productivity and transparency. Explore how PharmEng Technology can elevate your operations by visiting www.pharmeng.com or contacting us at info.asia@pharmeng.com. Let’s drive your success together!

About PharmEng Technology
PharmEng Technology is a global consulting firm specializing in pharmaceutical engineering, regulatory affairs, and compliance. With a commitment to quality and innovation, PharmEng Technology provides comprehensive solutions to meet the evolving needs of the pharmaceutical and biotechnology industries.
Contact Information
PharmEng Technology
Email: info.asia@pharmeng.com





