Digitizing CQV: Embracing the Future of Pharmaceutical Validation
by Dhika Prameswari and Rida Hadirah Ramli
Manufacturers are integrating digital transformation into Commissioning, Qualification, and Validation (CQV) processes to enhance productivity and mitigate risks in the life sciences industry, ensuring precision and compliance in pharmaceutical product safety and efficacy. As the industry advances, the integration of digital strategies into CQV is becoming not just an option, but a necessity for maintaining high standards and meeting the complex demands of modern pharmaceutical manufacturing.
Challenges in Traditional CQV
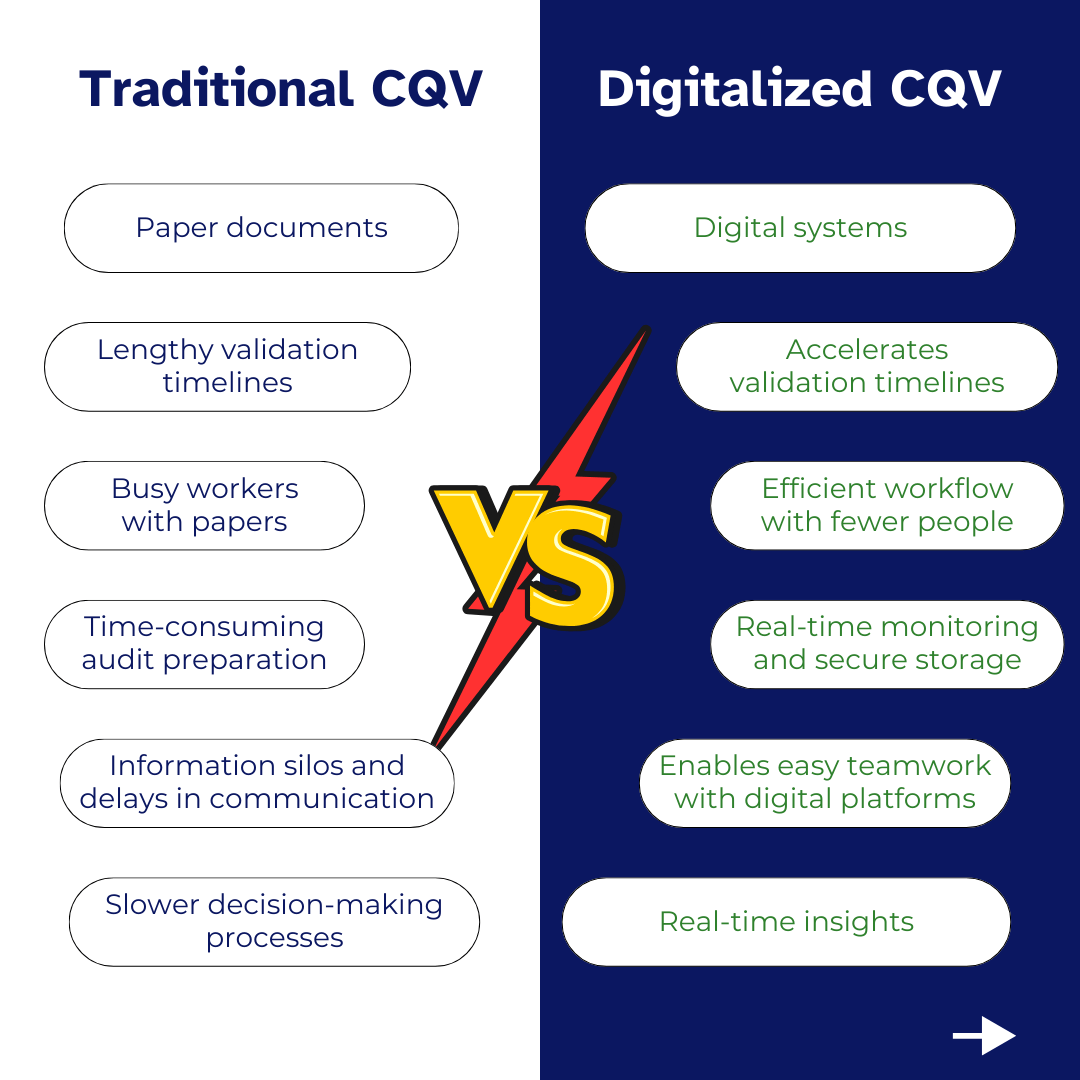
Commissioning and qualification are crucial stages in manufacturing equipment lifecycles, involving large amounts of data and information in paper-based documentation. Below is the comparison between traditional and digitalized CQV in manufacturing equipment lifecycles, highlighting key differences and benefits of digitalization:
|
Subject |
Traditional CQV |
Digitalized CQV |
|
Data Collection and Management |
Relies heavily on paper-based documentation |
Implements electronic data capture and management systems |
|
Validation Timelines |
Lengthy validation timelines |
Accelerates validation timelines |
|
Resource Utilization |
High resource consumption due to manual labour |
Optimizes resource utilization by focusing efforts on high-risk areas |
|
Compliance and Audit Readiness |
Significant time spent preparing for audits |
Real-time monitoring and secure storage of all documentation |
|
Collaboration and Communication |
Information silos and delays in communication |
Facilitates seamless collaboration through digital platforms |
|
Decision-Making |
Slower decision-making processes |
Real-time insights |
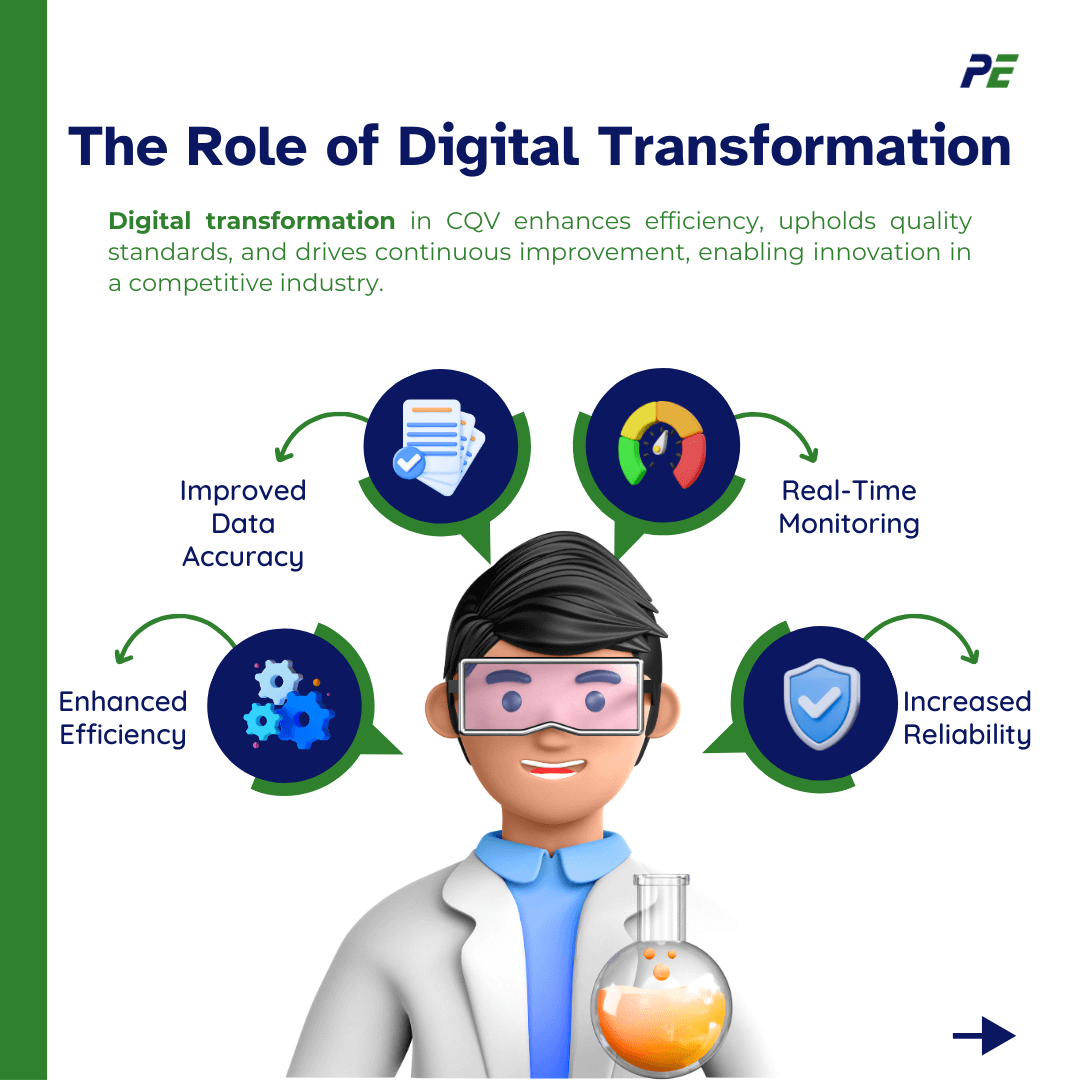
The Role of Digital Transformation
Digital transformation is indeed revolutionizing the Commissioning, Qualification, and Validation (CQV) processes in the pharmaceutical industry. Here is how:
- Improved Data Accuracy: Digital tools ensure that data used in CQV is precise and reliable, leading to more consistent outcomes and fewer discrepancies.
- Real-Time Monitoring: Digital transformation enables real-time monitoring, allowing for prompt issue resolution, reduced downtime, and enhanced overall process efficiency.
- Enhanced Efficiency Digital transformation speeds up product development and optimizes resource use, leading to faster market entry and significant cost savings.
- Increased Reliability: Advanced digital systems can identify potential issues before they become critical, enhancing the overall reliability of pharmaceutical manufacturing.
Digital transformation in CQV empowers companies to achieve greater efficiency, maintain high standards of quality, and foster continuous improvement, paving the way for innovation in an increasingly competitive industry.
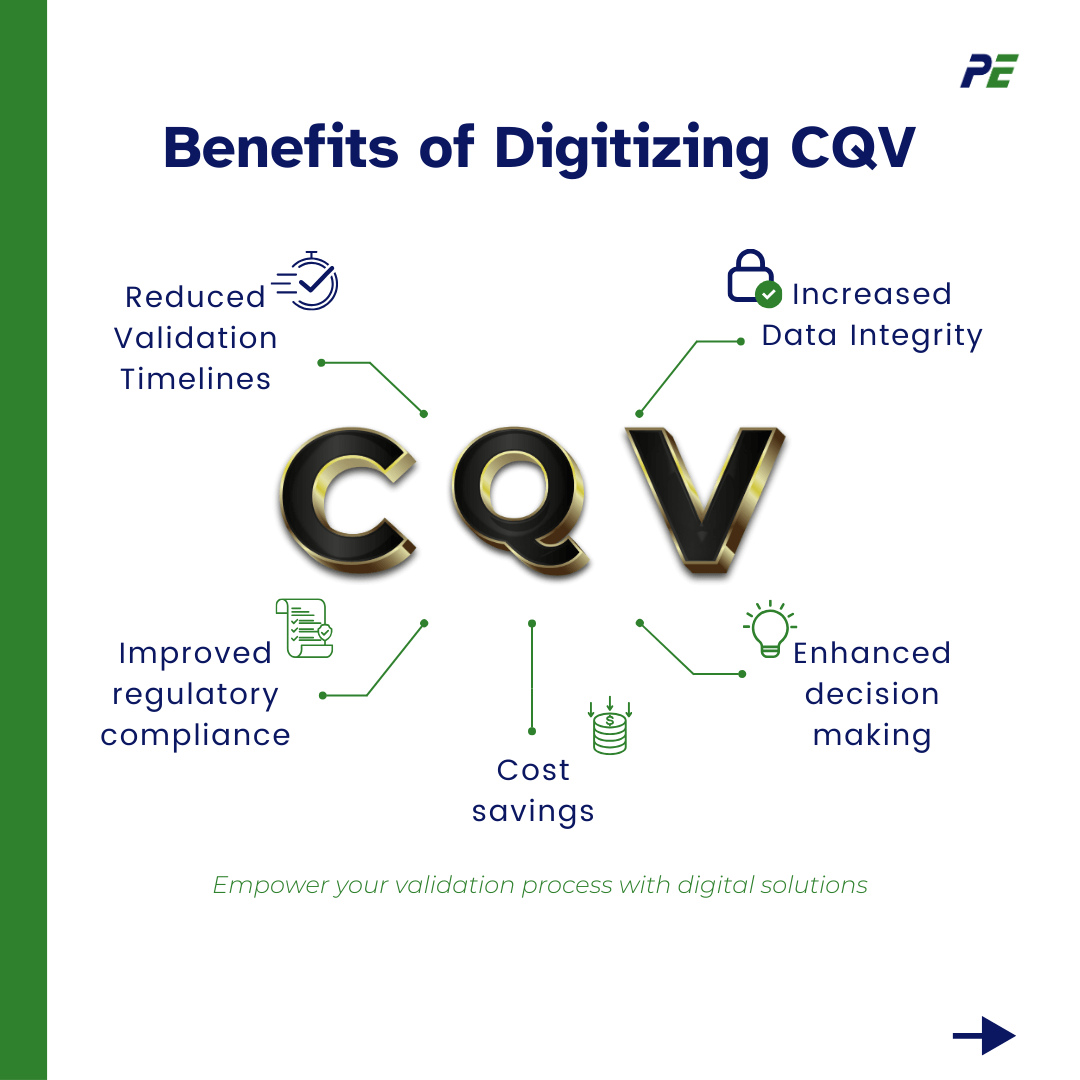
Benefits of Digitizing CQV
Digitalization has significantly impacted the way people work across various industries and sectors, including the pharmaceutical industry. Over the last few years, more pharma industries have been opened to digitalization. Here are several positive ways digitalization is transforming the pharmaceutical industry:
- Reduced validation timelines, monitoring systems in real-time help find and fix problems quickly, reducing testing time
- Increased data integrity, digital systems offer strong security features like encryption and access controls to protect important validation data
- Improved regulatory compliance, digital systems offer strong security, also create audit trails that track changes and access to data, improving traceability and accountability
- Cost savings, reduce manual labour in data entry, analysis, and documentation, leading to lower operational costs.
- Enhanced decision-making, digitization enable the use of advanced analytics tools to analyze large volumes of data, providing deeper insights into process performance and potential areas for improvement.
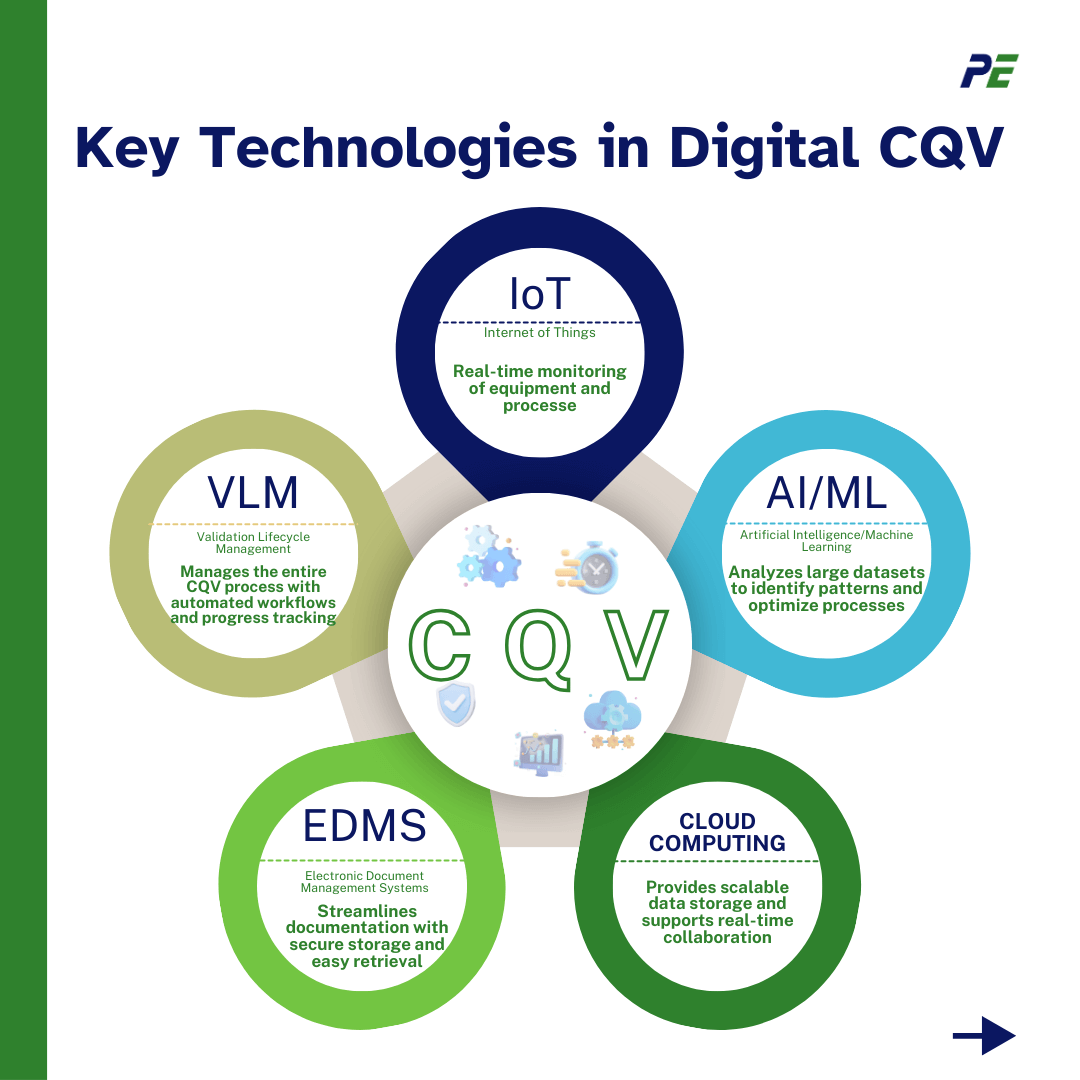
Key Technologies in Digital CQV
The digital transformation of Commissioning, Qualification, and Validation (CQV) in the pharmaceutical industry is being driven by several key technologies. Together, these technologies are creating a more connected, intelligent, and efficient CQV process that is better suited to the needs of modern pharmaceutical manufacturing. Here’s a look at the major technologies and their integration into CQV:
- IoT devices enable real-time monitoring of environmental conditions, equipment performance, and process parameters, enabling quick corrective actions, automated data collection, and predictive maintenance for potential failures.
- AI/ML algorithms analyze large datasets during CQV to identify patterns, trends, and anomalies, optimize processes, predict outcomes, and generate automated documentation and reports, ensuring consistency, accuracy, and regulatory compliance.
- Cloud computing platforms provide secure, scalable data storage, real-time team collaboration, and disaster recovery features for data integrity and regulatory compliance.
- EDMS (Electronic Document Management Systems) streamlines documentation by replacing paper with digital records, ensuring secure storage, easy retrieval, regulatory compliance, electronic signatures, and audit trails for data integrity.
- VLM (Validation Lifecycle Management) tools offer a comprehensive digital platform for managing the entire CQV process, automating workflows, monitoring progress, and facilitating seamless collaboration between teams and stakeholders.
Regulatory Considerations
As the pharmaceutical industry embraces digital CQV, it is crucial to carefully navigate regulatory guidelines from authorities like the FDA, EMA, and ISPE.
GAMP 5 (Good Automated Manufacturing Practice) and principles like ALCOA+ (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available), are transforming how pharmaceutical companies ensure quality and compliance.
GAMP 5 provides a risk-based approach to compliant computerized systems in the pharmaceutical industry, emphasizing the need for systems that are not only reliable but also capable of supporting continuous improvement. By aligning CQV processes with GAMP 5, companies can effectively manage the lifecycle of computerized systems, ensuring they meet the highest standards of quality and regulatory compliance.
ALCOA+ principles further enhance this approach by ensuring that data integrity is maintained throughout the CQV process. This framework demands that all data is Attributable, Legible, Contemporaneous, Original, and Accurate, with the additional focus on being Complete, Consistent, Enduring, and Available. These principles ensure that all electronic records and data generated during CQV are trustworthy and reliable, a critical factor in maintaining regulatory compliance.
Pharmaceutical companies can optimize their CQV processes by adopting digital transformation in line with GAMP 5 and ALCOA+ principles, ensuring regulatory compliance, performance optimization, and ongoing quality improvement and risk management.
Conclusion
In summary, digitizing CQV is a transformative step toward the future of pharmaceutical validation. By embracing digital tools and frameworks like GAMP 5 and ALCOA+ principles, companies can streamline processes, ensure data integrity, and enhance compliance with regulatory standards. This digital shift not only optimizes performance but also fosters a culture of continuous improvement and innovation. As the pharmaceutical industry evolves, digitizing CQV is not just an option—it’s a strategic imperative that enables companies to stay competitive, efficient, and at the forefront of quality assurance.
The Expertise of PharmEng Technology
At PharmEng Technology, we simplify the complexities of digitizing Commissioning, Qualification, and Validation (CQV) in the pharmaceutical industry. Our team of experts ensures your systems are compliant, efficient, and reliable, with deep knowledge of GAMP 5, ALCOA+ principles, and the latest digital technologies. We tailor our services to your needs, streamlining validation processes and maintaining top-quality standards.
Partner with PharmEng Technology to enhance your processes or embark on a digital transformation journey. We provide customized solutions for compliance, operational efficiency, and continuous improvement. Visit www.pharmeng.com or contact info.asia@pharmeng.com for more details.
About PharmEng Technology
PharmEng Technology is a global consulting firm specializing in pharmaceutical engineering, regulatory affairs, and compliance. With a commitment to quality and innovation, PharmEng Technology provides comprehensive solutions to meet the evolving needs of the pharmaceutical and biotechnology industries.
Contact Information
PharmEng Technology
Email: info.asia@pharmeng.com