Understanding CQV: The Backbone of Pharmaceutical Quality
by Dhika Prameswari and Rida Hadirah Ramli
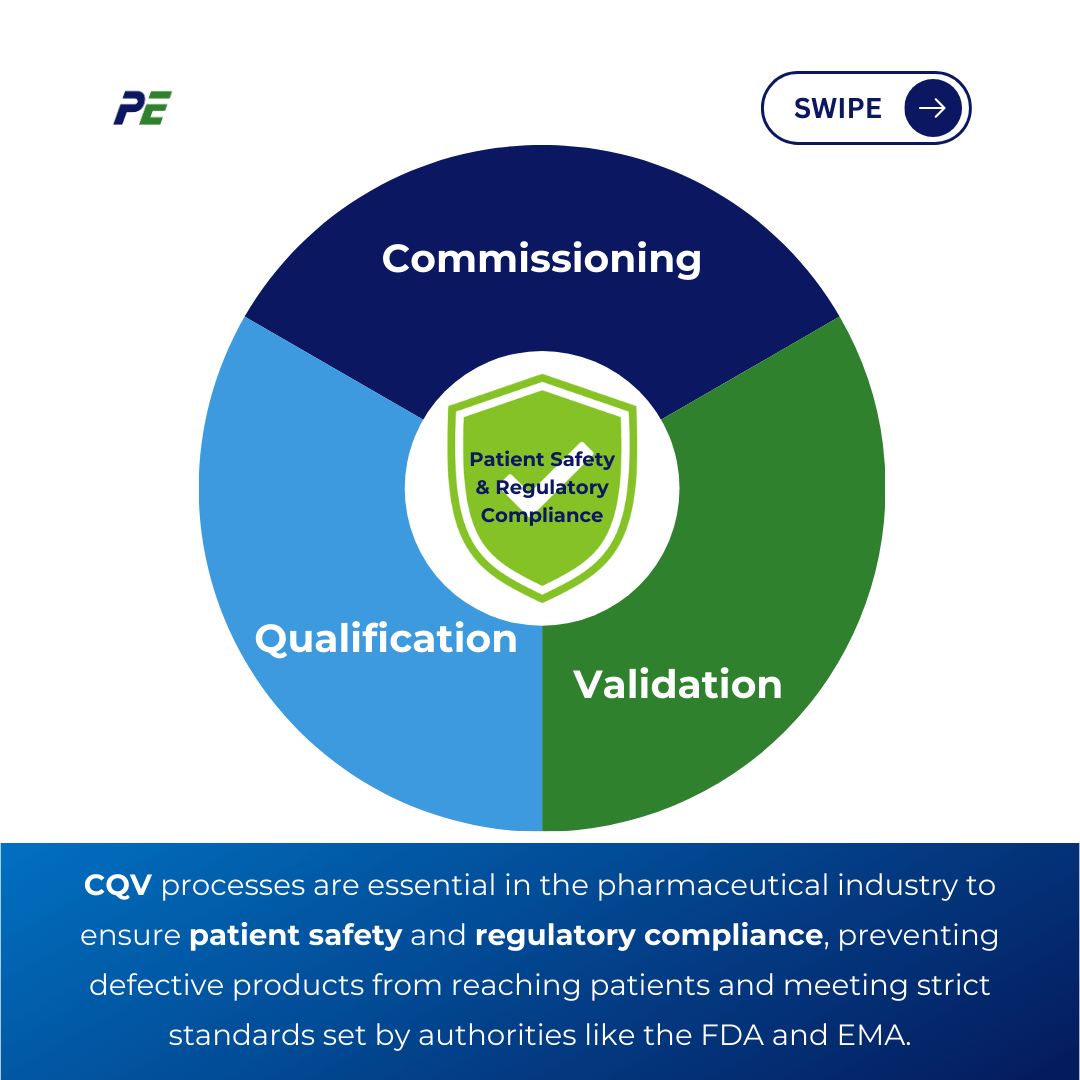
In pharmaceutical and medical device manufacturing, the concepts of Commissioning, Qualification, and Validation (CQV) form the backbone of ensuring that equipment, systems, and processes function as intended and consistently produce high-quality products. These activities are more than just operational necessities; they are stringent regulatory requirements designed to safeguard patient safety.
In the pharmaceutical industry, Commissioning, Qualification, and Validation (CQV) are critical processes that guarantee facilities, machinery, and processes are planned, built, tested, and maintained to provide high-quality products that satisfy regulatory standards.
The following are crucial components of CQV in the pharmaceutical sector:
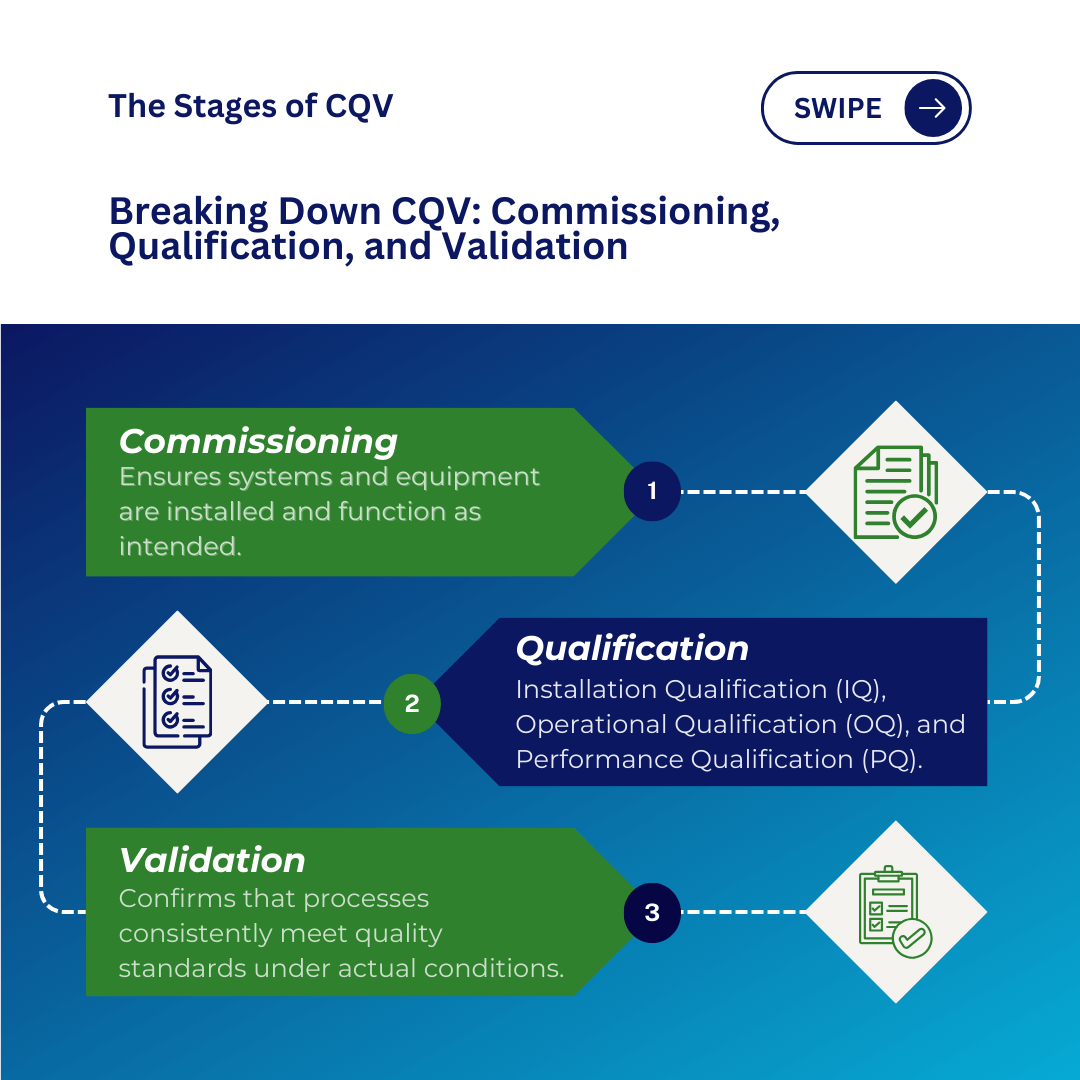
Commissioning is a systematic process that ensures facilities, systems, and equipment are installed, tested, and operationally conform to user needs and design specifications.
Qualification refers to the process of ensuring that equipment, systems, and facilities are properly installed, operate as intended, and perform consistently to produce high-quality products that meet predefined specifications and regulatory standards.
The qualification process is typically divided into three pivotal stages:
- Installation Qualification (IQ): Verifies that equipment and systems are installed according to design and manufacturer specifications.
- Operational Qualification (OQ): Tests the equipment or system to ensure that it operates within the intended operating ranges.
- Performance Qualification (PQ): Confirms that the equipment, systems, or processes consistently perform as intended under simulated real-world conditions.
Each qualification phase is instrumental in ensuring the integrity of manufacturing processes and the quality of the final product.
Validation ensures that systems, processes, or equipment consistently produce results that meet quality standards under actual operating conditions, confirming that the entire production process, from raw materials to final product, operates as intended and produces safe, high-quality products.
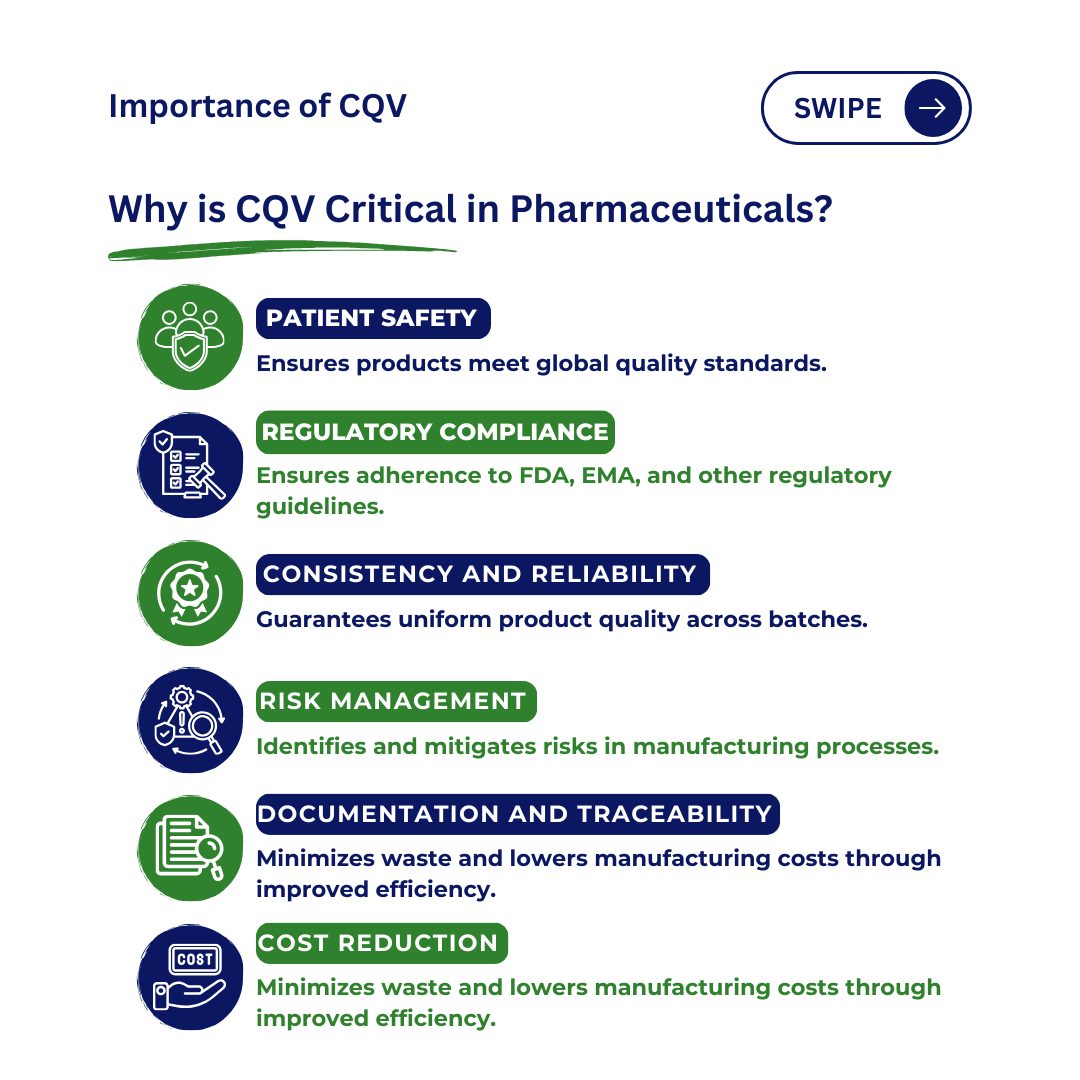
Why are Commissioning, Qualification, and Validation important?
- Patient Safety: Patient safety is the primary concern in pharmaceutical and medical device manufacturing, and qualification and validation processes guarantee that equipment meets global quality and regulatory standards, lowering the risk of defective products.
- Regulatory Compliance: The pharmaceutical and life sciences industries are regulated by authorities like the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA), which ensure that products meet quality standards and safety requirements.
- Consistency and Reliability: Qualification and validation processes guarantee that production processes are consistent, product variability is minimized, and pharmaceutical product batches fulfill the specified requirements.
- Risk Management: Qualification and validation are crucial in identifying potential risks and vulnerabilities in manufacturing processes, thereby mitigating risks related to product quality, safety, and compliance.
- Documentation and traceability: The qualification and validation process necessitates thorough documentation and traceability of all activities, proving regulatory compliance and providing a clear record for audits or investigations.
- Cost reduction: The initial investment in qualification and validation can lead to long-term benefits such as reduced batch rejections, product recalls, and improved process efficiency, resulting in cost savings.
The FDA is responsible for ensuring proper validation practices as outlined in 21 CFR sections 210 and 211. Additionally, the FDA provides guidance documents that interpret federal codes and offer further context and depth. For example, the 2011 FDA guidance on process validation gives a practical framework and explains how its components align with regulatory requirements.
On the other hand, ICH Q9 emphasizes the importance of quality systems in the pharmaceutical industry, recognizing CQV as a critical element of an effective quality system. Integrating CQV into these systems helps to identify, assess, and control any potential risks to product quality, thereby enhancing overall compliance, consistency, and reliability in pharmaceutical manufacturing. This proactive approach ensures that potential issues are managed effectively, contributing to the continuous improvement of quality processes and the protection of patient safety.
Conclusion
In conclusion, Commissioning, Qualification, and Validation (CQV) form the backbone of pharmaceutical quality, playing an essential role in the successful execution of pharmaceutical projects. CQV professionals are crucial in ensuring that new facilities or medical devices are constructed and operated in compliance with regulatory requirements, safety standards, and product quality specifications. Their expertise is integral to the successful launch and continuous operation of the pharmaceutical processes, safeguarding both the integrity of the processes and the quality of the final products.
Partnering with PharmEng Technology
At PharmEng Technology, we recognize the critical importance of CQV in the pharmaceutical and life sciences industries. Our team of experts is dedicated to helping your organization navigate the complex regulatory landscape and implement robust CQV practices. Whether you are launching a new facility, upgrading existing systems, or seeking to optimize your processes, PharmEng Technology offers tailored solutions that ensure compliance, enhance efficiency, and safeguard product quality.
Partner with PharmEng Technology to access top-notch expertise and innovative solutions that ensure regulatory compliance and drive continuous improvement. Together, we’ll build a foundation of quality and reliability for your pharmaceutical projects. Visit us at www.pharmeng.com or email us at info.asia@pharmeng.com for more information.
About PharmEng Technology
PharmEng Technology is a global consulting firm specializing in pharmaceutical engineering, regulatory affairs, and compliance. With a commitment to quality and innovation, PharmEng Technology provides comprehensive solutions to meet the evolving needs of the pharmaceutical and biotechnology industries.
Contact Information
PharmEng Technology
Email: info.asia@pharmeng.com