QA vs QC in Pharmaceutical Industry
by Roseline Tio, Dhika Prameswari and Izwan Firdaus (SEA CSV SME)
It’s a common question: quality assurance vs. quality control in the pharmaceutical industry. What are the differences, and why are they important?
In this guide, we’ll explore the differences in quality assurance vs. quality control, defining both terms and discussing their advantages before we move on to discuss how QC and QA in pharmaceuticals works.
Quality Assurance vs Quality Control: Definition
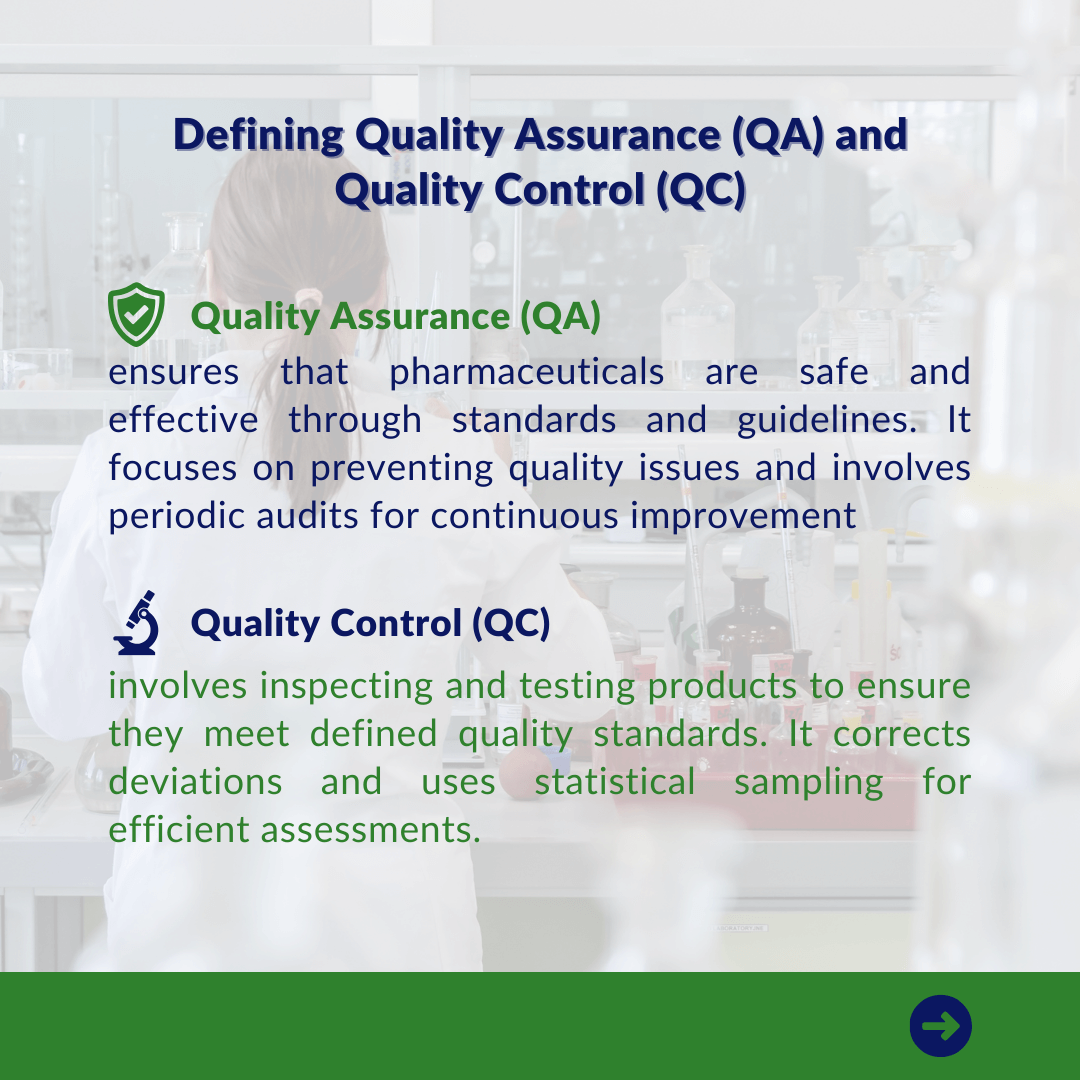
Quality assurance (QA) is a critical component of the pharmaceutical industry because it gives consumers confidence that the pharmaceuticals and therapeutic drugs, they need to be healthy are safe, and that the manufacturing and drug development processes are cost-effective and profitable. It is also critical in the regulatory affairs environment for pharmaceuticals industry, assisting organisations in obtaining regulatory approval to launch new products.
QA creates standards and guidelines that serve as benchmarks for expected quality levels and ensure that the team works toward a common goal. Periodic audits are undertaken to improve and ensure adherence to standards, contributing to a continual culture of process improvement.
Quality control (QC) refers to the actions and techniques used to check and test the final product for compliance with defined quality standards. It entails thoroughly inspecting and testing against predetermined criteria to detect and correct deviations from quality standards. Statistical sampling techniques, such as random sampling, are used to provide valid assessments without scrutinizing each item, hence increasing efficiency. During QC, faults are corrected immediately, and the process may entail numerous iterations to ensure continual quality improvement.
QC involves extensive documenting of inspection results for record-keeping, which serves as a feedback loop for future process improvements. Furthermore, quality control assures compliance with set standards and is critical for achieving client expectations, which contributes to total customer satisfaction.
How QA and QC in Pharmaceutical works
Now that we’ve understood the debate around quality assurance vs. quality control, we can closely examine how the process actually works within the pharmaceutical industry.
QA in Pharmaceuticals
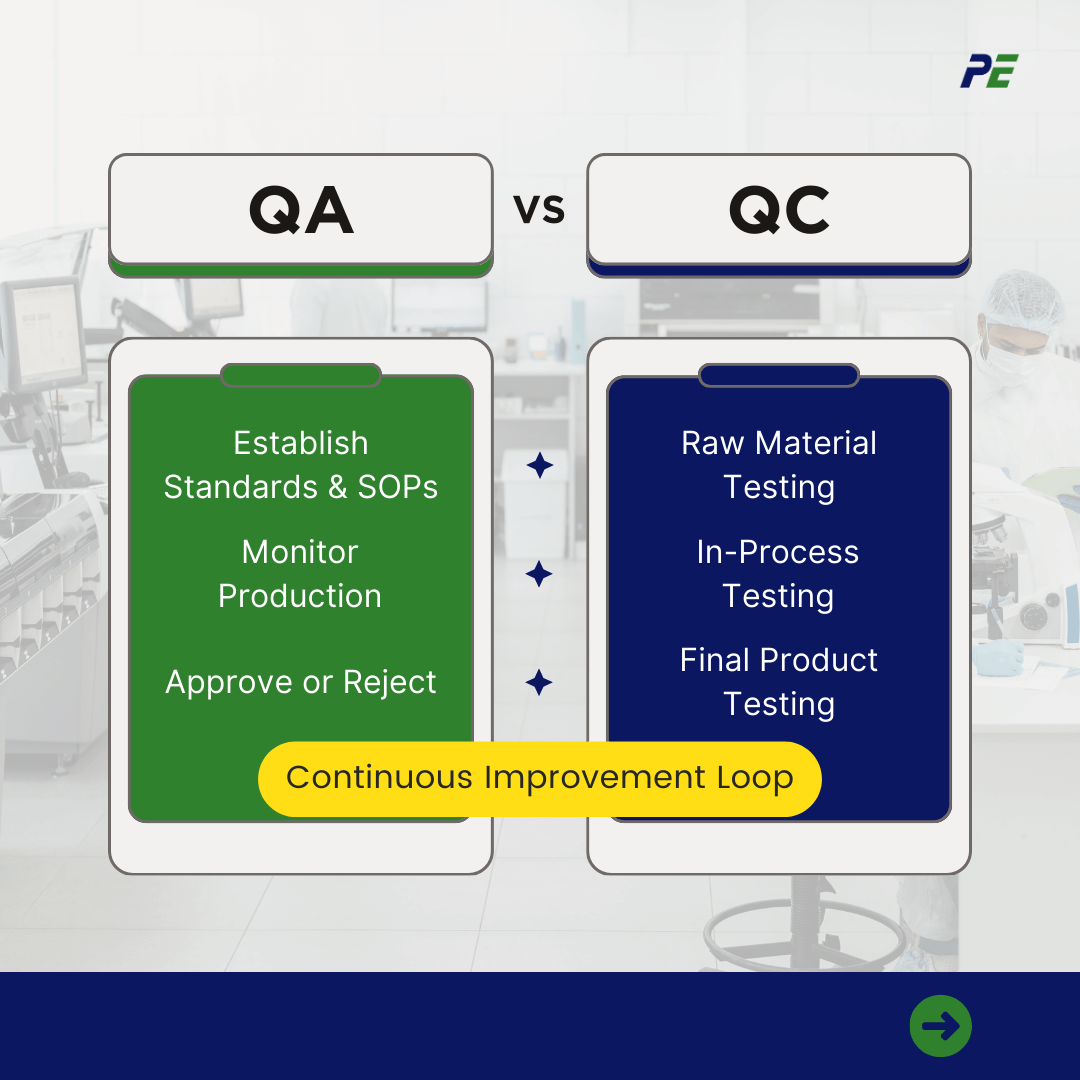
QA in pharmaceuticals is process-oriented and focuses on preventing quality issues before they occur—of course, not much can be done to prevent all quality issues due to humans dependent and the manufacturing environment are both fallible, which is where QC comes in.
Quality assurance frameworks are often created as a set of standard operating procedures (SOPs) that align with the ISO 13485:2016 principles on quality management systems. In short, these ensure:
- Pharmaceutical organizations pursue sustained growth by implementing a strong quality management system.
- Customers and healthcare practitioners can be satisfied in a company’s capacity to produce consistent and safe products that comply to their defined specifications.
- Both employees and corporate executives can benefit from excellent management training, learning, and professional development.
QC in Pharmaceuticals
QC identifies quality issues in pharmaceutical drugs, ensuring they meet safety-critical standards and consumer satisfaction standards, ensuring products meet established quality assurance framework standards.
Quality control in the pharmaceutical industry has a number of steps, including:
- Quality control allows organizations to conduct QC tests at any stage of the manufacturing process, ensuring the quality of raw materials before production, thereby minimizing waste.
- In-process Control testing ensures pharmaceutical drug manufacturing adheres to guidelines, while finished product tests ensure product quality before storage, shipping, and distribution to consumers.
- Ensures traceability of all samples and drugs tested, providing accurate records for consumers, healthcare providers, and regulators to access.
Key Takeaways
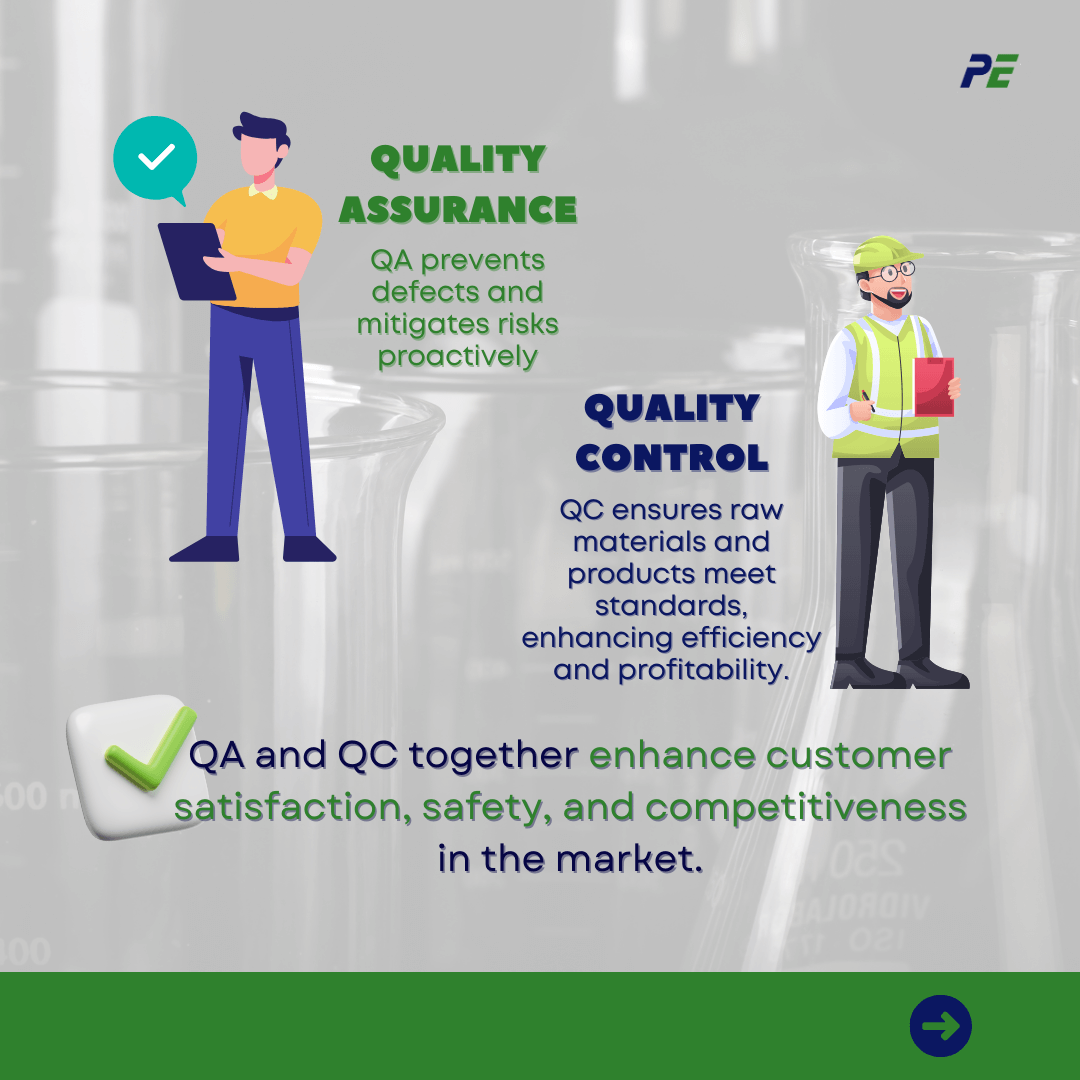
Pharmaceutical quality assurance (QA) proactively prevents defects and mitigates risks, while quality control ensures raw materials and finished products meet quality assurance standards.
Quality control enhances process efficiency, identifies improvement areas, streamlines operations, and increases profitability by bridging established standards with early production inspection, reducing waste and litigation risks.
QA and QC work together in pharmaceutical quality management, enhancing customer satisfaction, maintaining safety reputation, and maintaining competitiveness in the rapidly evolving market.
Encouraging both Quality Control and Quality Assurance (QC) and Quality Assurance (QA) in pharmaceuticals is crucial for building trust with consumers, healthcare professionals, and regulatory agencies.
Partnering with PharmEng Technology

PharmEng Technology‘s consultants are dedicated to transforming people’s lives and putting outstanding, motivated individuals at the leading edge of the life sciences business. Whether you’re looking for a job or an executive to lead your company and ensure its competitiveness, we have access to the world’s top opportunities.
For further details, please visit our website at www.pharmeng.com or contact us via email at info.asia@pharmeng.com. Discover how PharmEng Technology can assist you in reaching your validation objectives and fostering success in the pharmaceutical sector.
About PharmEng Technology
PharmEng Technology is a global consulting firm specializing in pharmaceutical engineering, regulatory affairs, and compliance. With a commitment to quality and innovation, PharmEng Technology provides comprehensive solutions to meet the evolving needs of the pharmaceutical and biotechnology industries.
Contact Information
PharmEng Technology
Email: info.asia@pharmeng.com





