
How to Implement PDCA Cycle
by Roseline Tio, Dhika Prameswari
The PDCA cycle is a systematic process of planning, doing, checking, and acting, facilitating effective problem-solving and change management, particularly useful for small-scale improvement testing.
The PDCA cycle offers a systematic and continual approach for dispute decision, decision making, and continuous improvement. In this post, we will look at what the PDCA cycle is and how it is used.
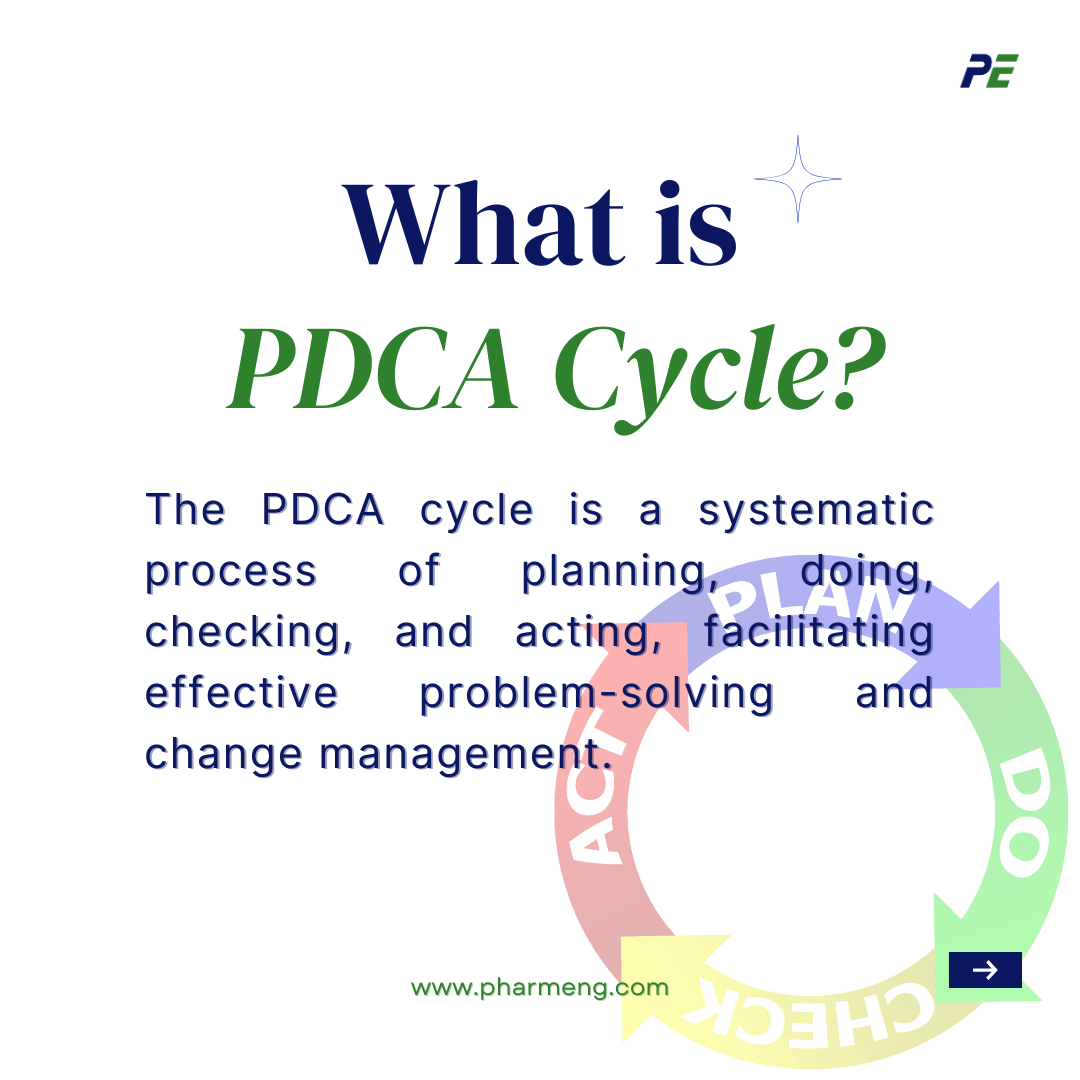
What is PDCA Cycle?
The PDCA cycle is a four-step iterative process that helps you solve problems and implement changes in your manufacturing operations. This cycle is based on the scientific method and the principle of continuous improvement. Here is a breakdown.
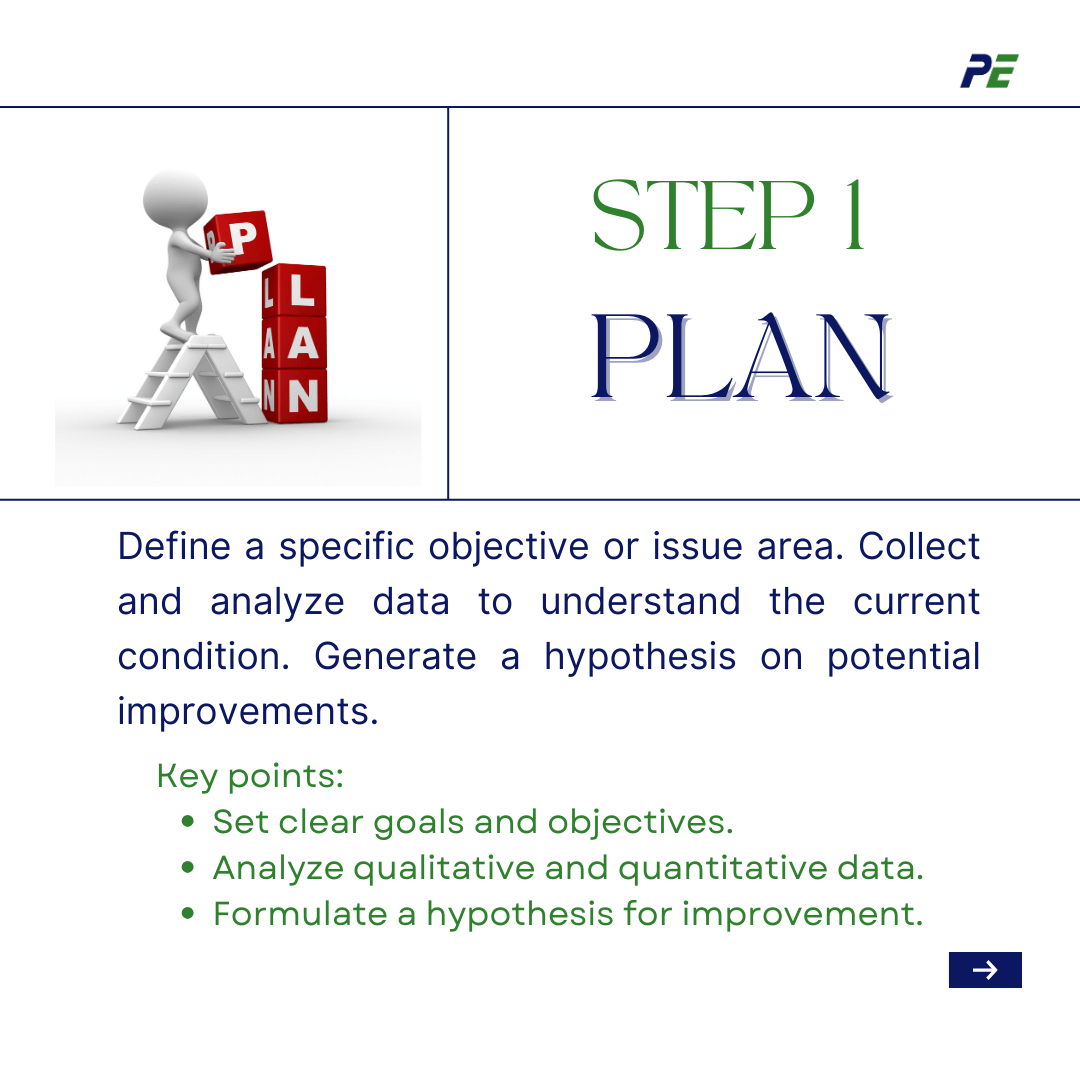
- Plan
Starting by defining a specific objective or issue area in your business that you would like to address. Collect and analyse data for improved comprehension of the present condition of the process or product. This often includes qualitative or quantitative data, ranging from consumer feedback to performance measures. Based on the data analysis, a hypothesis will be generated to determine what adjustments may enhance the process.
- Do
There is where the plan is put into action. You will do Implement a small-scale pilot program to minimize potential issues and risks. Document during Do phase such as collect data, document process deviations, and observations for analysis and all data collected will be analysed in Check phase.

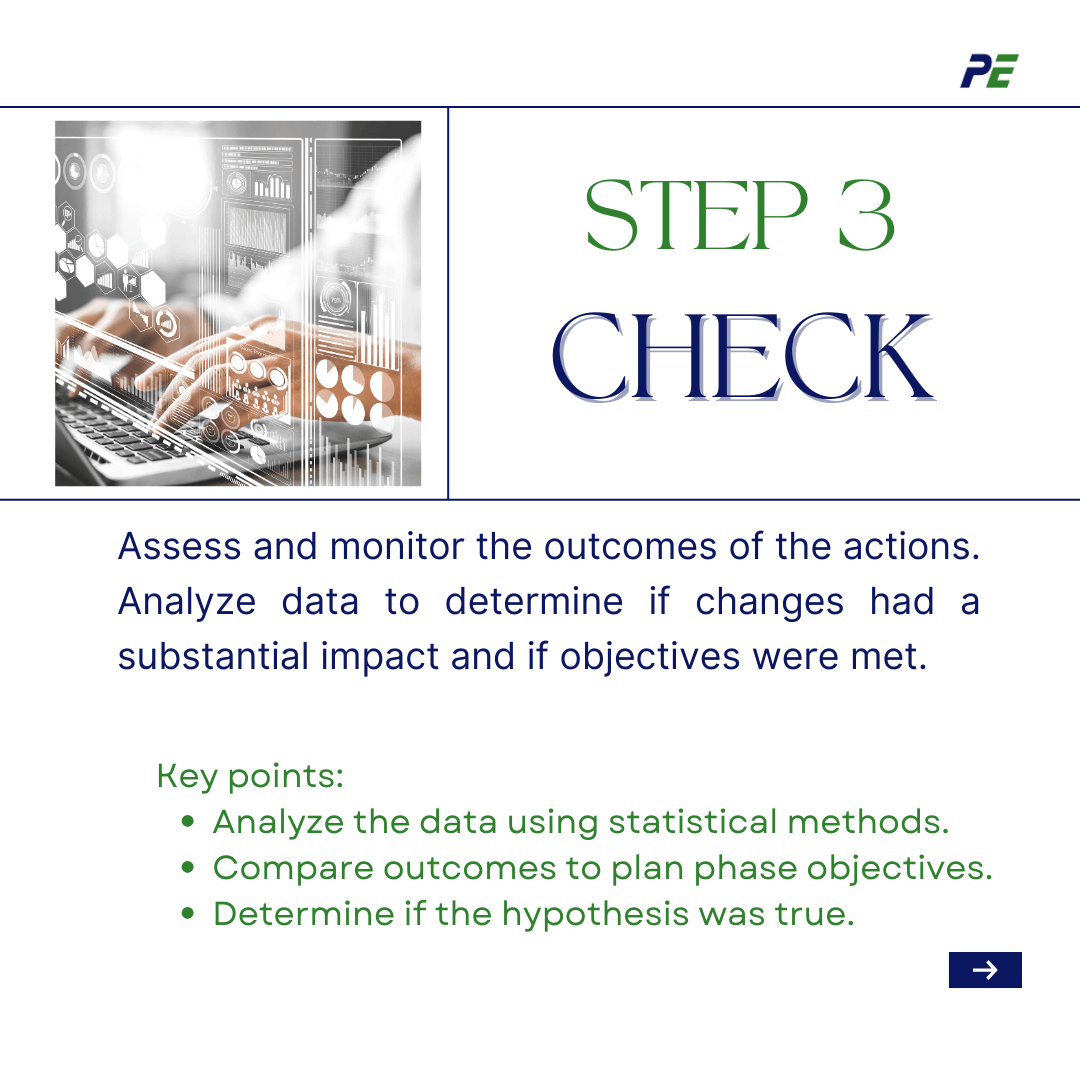
- Check
This step involves assessing and monitoring the outcomes of the executed actions. You will analyse the data acquired during the Do phase using statistical methods to assess if the changes had a substantial impact. The plan phase objectives were established, and the outcomes were analysed to see whether the hypothesis was true and the objectives were met. The analysis should be completed, and if positive, the Act phase should be launched, otherwise, the causes and other solutions should be investigated.
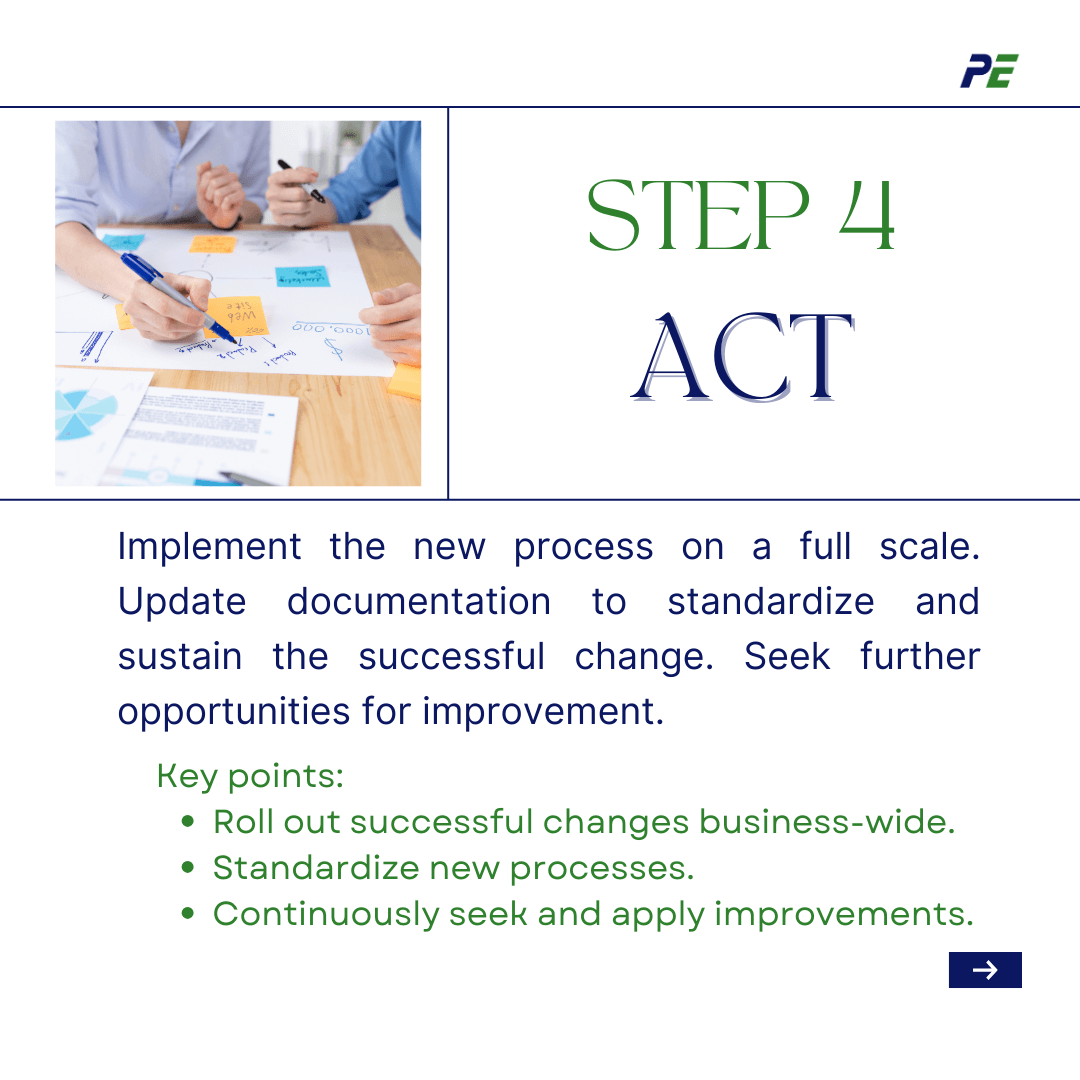
- Act
Based on Check phase result, you will take action. The new process should be implemented on a full scale after the Check phase. To ensure consistency across the business, it is essential to update the process documentation to standardize and sustain the successful change. The cycle of improvement involves seeking opportunities for improvement, refining plans based on learnings, and applying them again if necessary.
How it is used?
The PDCA cycle is a continuous improvement methodology that employs an iterative approach. The processes are repeated cyclically, allowing for continuous improvement and advancement. Each cycle builds on the information and experience gained in the preceding one, resulting in constant progress. The PDCA cycle may be used by organizations at all levels, from individual projects to whole business processes, to improve efficiency, quality, and overall performance.
The PDCA cycle is an excellent tool for promoting continual development and success. Organizations may identify areas for improvement, make changes, assess outcomes, and enhance processes by iterating through the phases Plan, Do, Check, and Act. The PDCA cycle encourages a culture of learning, experimentation, and flexibility, allowing firms to fulfil their objectives, improve customer satisfaction, and remain ahead in today’s competitive environment.
Your Trusted Partner

No matter where you work or what your problem-solving position entails, PharmEng Technology is a problem-solving consultant who will help your team figure out why a method, strategy, or project isn’t operating as expected.
At PharmEng Technology, our engineer has extensive experience in problem solving management to provide best decision. Our engineers meticulously monitor each stage of the project to ensure adherence to current regulatory and quality standards through rigorous data collection.
For further details, please visit our website at www.pharmeng.com or contact us via email at info.asia@pharmeng.com . Discover how PharmEng Technology can assist you in reaching your validation objectives and fostering success in the pharmaceutical sector.
About PharmEng Technology
PharmEng Technology is a global consulting firm specializing in pharmaceutical engineering, regulatory affairs, and compliance. With a commitment to quality and innovation, PharmEng Technology provides comprehensive solutions to meet the evolving needs of the pharmaceutical and biotechnology industries.
Contact Information
PharmEng Technology
Email: info.asia@pharmeng.com





