
Choosing a Computerized System Validation Partner
by Roseline Tio, Dhika Prameswari, Izwan Firdaus (SEA CSV SME)
Current Good Manufacturing Practices (cGMP) encourage Life-Science Industry to stay and adapt the current trend of technology which soon will unshackle the human dependent process. The introduction of Pharma 4.0 in 2017 by ISPE, the Life-Science Industry had experienced the paradigm shift of the manufacturing process towards digitalization. The implementation of digitalization in compliance manner is not able to escape from the Computerized System Validation (CSV) activities.
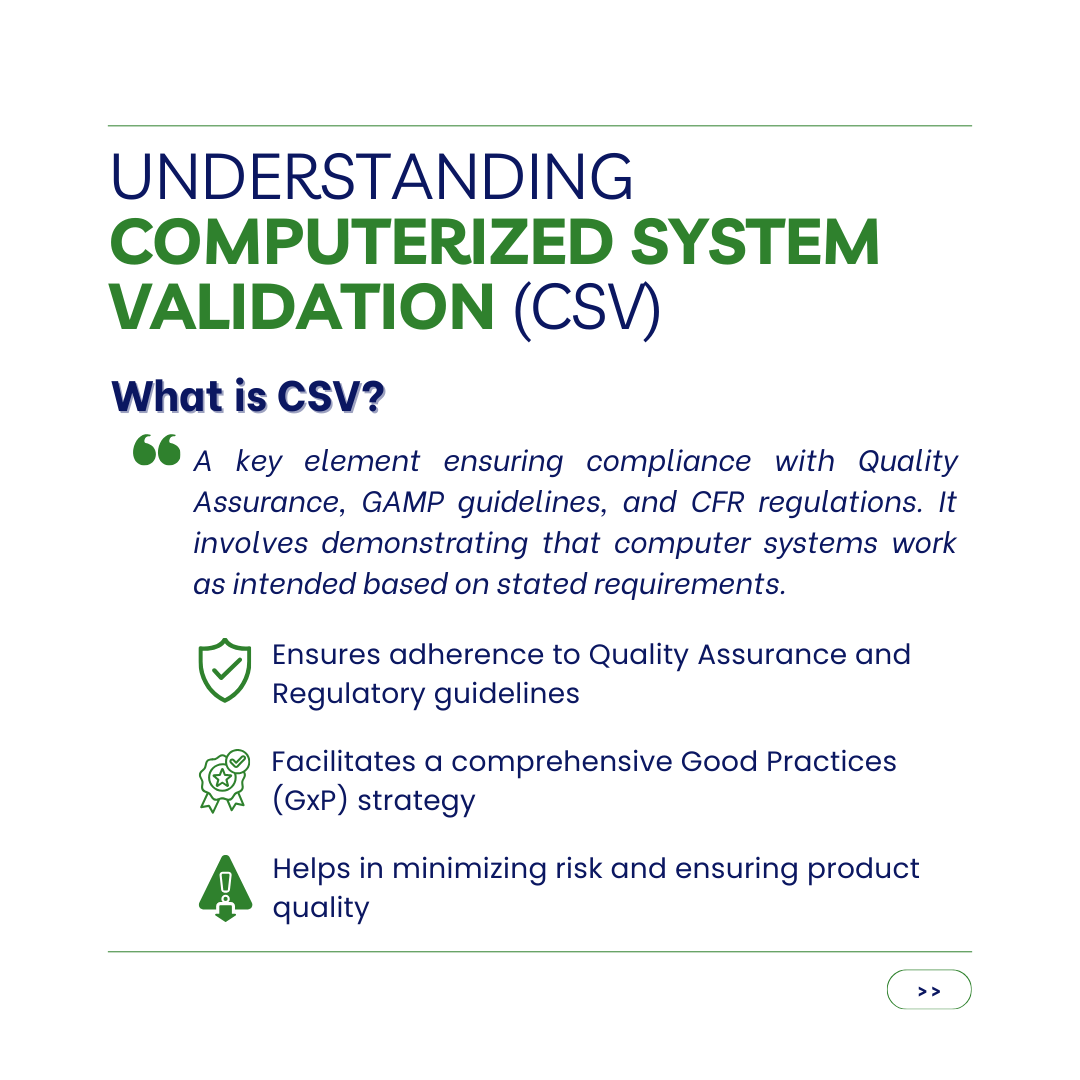
CSV is a key element of ensuring the Quality Assurance and the GAMP (Good Automated Manufacturing Practices) guidelines, as well as the Code of Federal Regulations (CFR) are complied. Computerized System Validation (CSV) involving the demonstrating and documenting that respective computer systems work as intended use based on a set of stated requirements. Computer system validation allows Life-Science industry creating a comprehensive a Good Practices (GxP) strategy to decrease risk and guarantee products are consistently delivered to high quality standards. Digesting the FDA’s huge amount of regulatory requirements on this issue can be a daunting undertaking, thus it is strongly advised to select an experienced computerized system validation partner who can guide you through this complicated yet crucial field.
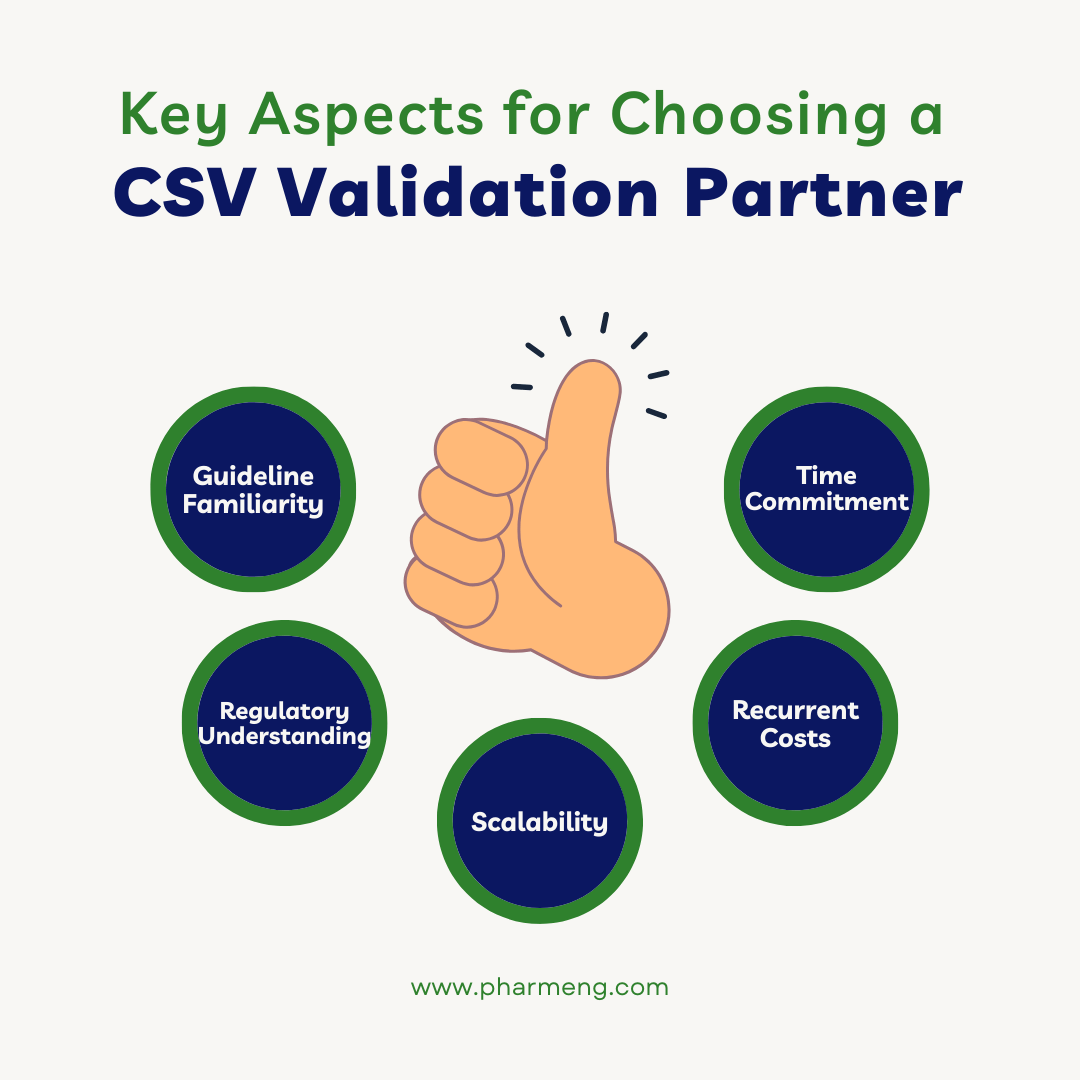
Below are the key aspects for choosing validation partner for CSV:
- Is your GxP validation partner intimately aware with guideline materials such as ISPE’s GAMP5 risk-based approach to GxP computer system validation?
- Does your GxP validation partner understand the FDA’s Title 21 CFR Annex 11 rules regarding electronic records and electronic signatures? Title 21 CFR Annex 11 establishes the set of regulations that apply to electronic documents and electronic signatures as if they were paper records.
- What is your partner’s time commitment for implementation? Does it take months, weeks, or days to get started?
- What are the recurrent costs? Do they have a strategy if the work exceeds the scope?
- What about scalability? Is your partner supplied with additional resources that are already trained?
Our Expertise
To guarantee a complete grasp of compliance, our team thoroughly reviews important regulatory regulations, such as FDA 21 CFR Part 11 and EU Annex 11. We thoroughly review their regulations to discover particular needs for CSV operations such as data integrity, electronic records, and electronic signature. We evaluate the client’s existing systems, procedures, and documentation to ensure compliance with regulatory requirements.
In order to collaborate with the client, our team creates a thorough roadmap outlining the sequential processes, milestones, and schedule for CSV implementation, guaranteeing transparency and responsibility throughout the process.
In the other hand, we engage with stakeholders across different departments and level of the organization to foster understanding, alignment, and commitment to the CSV strategy.
We educate stakeholders on CSV concepts, methodology, and best practices through interactive workshops and seminars, providing them with the information and skills required to implement effectively.
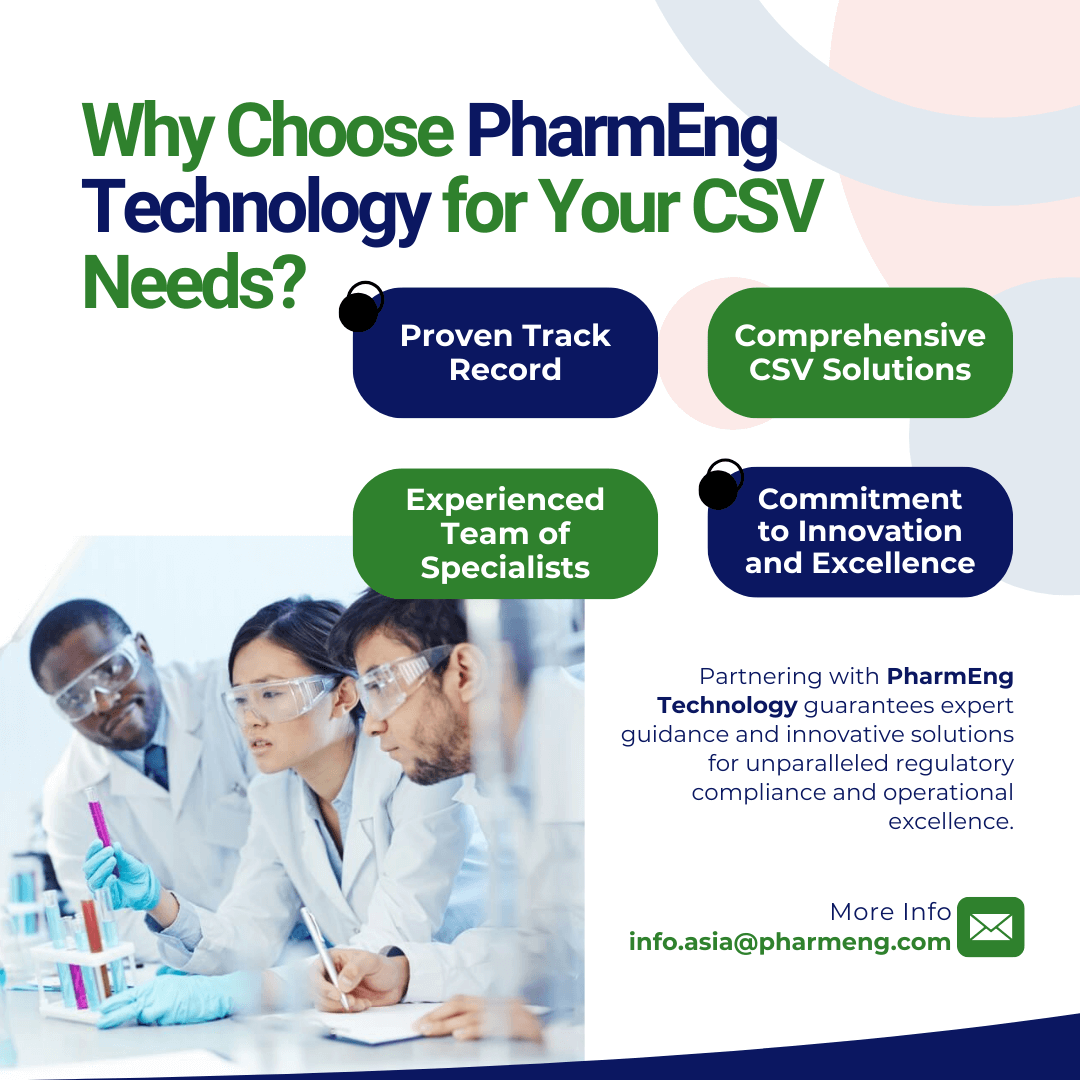
The Expertise of PharmEng Technology
At PharmEng Technology, our experienced specialists contribute knowledge, insight, and a proven track record of assisting firms through the difficulties of regulatory compliance. We are devoted to assisting our clients in achieving and maintaining compliance, improving patient safety, and driving operational excellence in the pharmaceutical business.
Partnering with PharmEng Technology
Partnering with PharmEng Technology means going on a path of pharmaceutical compliance, innovation, and success. Our persistent commitment to excellence, devotion to offering high-quality services, and track record of accomplishment make us the ideal partner in your pursuit of regulatory compliance and operational efficiency.
In a rapidly evolving marketplace where technology advances continue to change the pharmaceutical sector, taking a forward-thinking approach to CSV Strategy execution is critical. Organizations who work with PharmEng Technology may maximize the potential of their operations, promote compliance, and position themselves for long-term success.
For further details, please visit our website at www.pharmeng.com or contact us via email at info.asia@pharmeng.com. Discover how PharmEng Technology can assist you in reaching your validation objectives and fostering success in the pharmaceutical sector.
About PharmEng Technology
PharmEng Technology is a global consulting firm specializing in pharmaceutical engineering, regulatory affairs, and compliance. With a commitment to quality and innovation, PharmEng Technology provides comprehensive solutions to meet the evolving needs of the pharmaceutical and biotechnology industries.
Contact Information
PharmEng Technology
Email: info.asia@pharmeng.com





