From Compliance to Confidence: The Role of Computerized System Validation
by Roseline Tio, Dhika Prameswari, Izwan Firdaus (SEA CSV SME)
What is Computerized System Validation?
Computerized System Validation (CSV) is a systematic and documented method for ensuring that a computerized system, such as software or hardware, continuously functions as intended while fulfilling specified specifications and regulatory requirements. The term is used to cover a broad range of systems, including automated manufacturing equipment, control systems automated laboratory systems, and manufacturing database systems. It is a key quality assurance technique, especially in sectors that rely heavily on computerized technology.
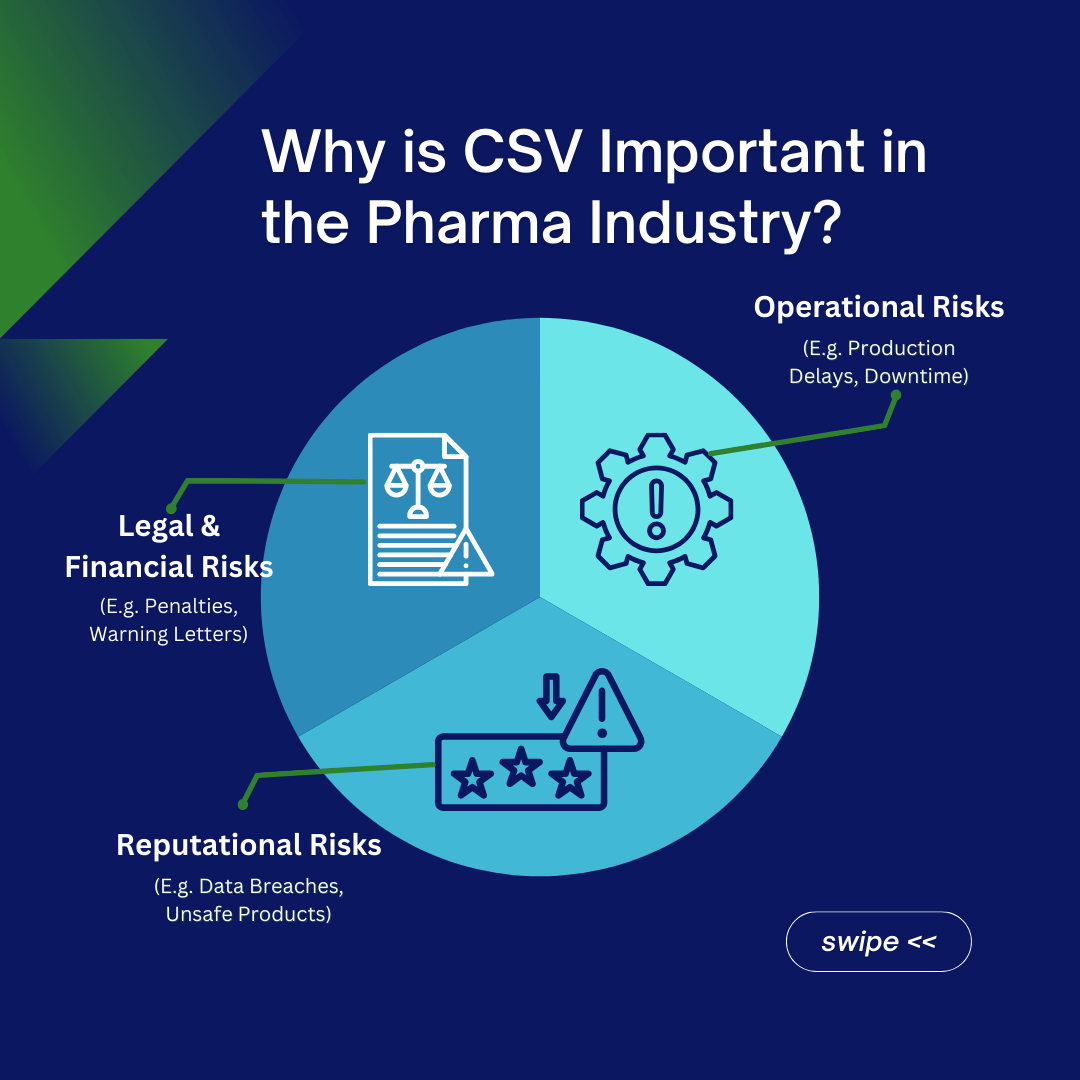
Why is CSV Important in the Pharma Industry?
CSV is necessary for businesses where computer system stability and accuracy are critical, particularly in pharmaceutical manufacture, clinical research, and healthcare, where high regulatory criteria must be met. Failure to adopt CSV in regulated businesses can result in a variety of problems, including legal, financial, operational, and reputational penalties. Regulatory authorities, like the FDA, EMA, and others, require companies to evaluate computer systems to ensure data integrity, security, and dependability. Noncompliance with these regulations can result in regulatory actions such as penalties, warning letters, and product recalls.
Without effective computer system validation in the pharmaceutical business, there is a higher chance of data integrity issues such as inaccuracies, inconsistencies, and illegal access or data alterations. This can jeopardize the accuracy and dependability of data utilized in essential procedures.
In businesses such as pharmaceuticals, failure to validate computer systems in manufacturing or quality control procedures can result in the creation of poor or hazardous products, endangering patient health and safety.
Unvalidated systems are more susceptible to errors, failures, and disruptions. This can cause downtime, production delays, and challenges in managing business operations, resulting in financial losses. Inefficient or unstable systems can reduce production and workflow efficiency. This can have an impact on overall business performance and competitiveness. Unvalidated systems may also lack strong security measures, leaving them vulnerable to cyber threats and unauthorized access. This can result in data breaches, sensitive information loss, and reputational damage.
Companies that operate in regulated industries are audited by regulatory organizations. Pharmaceutical businesses that fail to validate our computer systems may face higher attention during audits, perhaps leading to additional investigations and penalties.
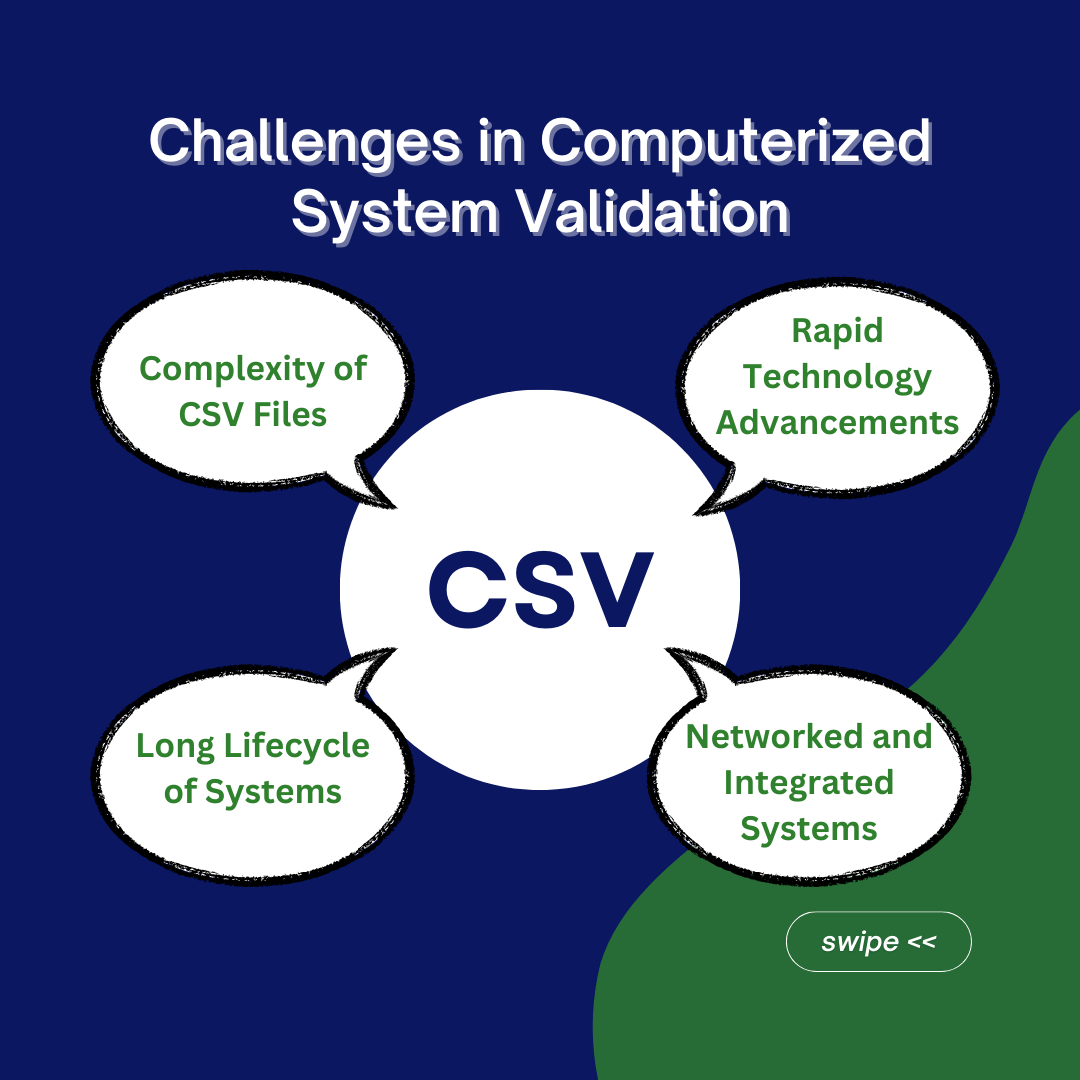
Without proper computer system validation in pharmaceuticals, establishing best practices and making changes to computer systems becomes difficult. This can stifle innovation and adaption to new technologies, placing the business at a disadvantage. The industry has viewed this process as fairly difficult for a variety of reasons. CSV files can be quite complex. The particular properties of the systems being validated, as well as the regulatory framework in which they operate, frequently contribute to this complexity. Technology advances quickly, making it difficult to stay current while adhering to existing rules. The introduction of new technologies, software, and platforms complicates the validation process.
Organizations employ computerized systems for a variety of objectives, including manufacturing, quality control, data management, and reporting. Each sort of system may have different characteristics and validation requirements. Many of these computer systems are networked and integrated into modern businesses. Validating one system frequently necessitates consideration of its relationships with other systems, making the validation process more complex. Computer systems typically have long lifecycles. Validating a system at implementation is only the beginning; sustaining validation throughout the system’s lifecycle offers continual problems, particularly with upgrades and revisions.
The integrity of data generated and handled by computer systems is an important feature of CSV. Managing data across several processes while avoiding mistakes, corruption, and unauthorized access increases complexity. Despite these obstacles, CSV is critical for guaranteeing the dependability, correctness, and compliance of computer systems in regulated sectors.
PharmEng Technology is a leading service provider in the field of Computerized System Validation, renowned for its comprehensive and meticulous approach. Here’s why our services stand out:
- Expertise and Experience: With years of experience in the industry, PharmEng Technology has developed a deep understanding of the regulatory requirements and technical intricacies involved in CSV. Our team of experts is adept at navigating the complexities of validation processes to ensure full compliance
- Customized Solutions: Recognizing that each client has unique needs, PharmEng Technology offers tailored validation solutions. They assess the specific requirements of each system and design validation protocols that are both efficient and compliant with industry standards.
- End-to-end Support: From initial planning to final execution, PharmEng Technology provides end-to-end support for CSV projects. Our comprehensive services include risk assessments, validation planning, protocol development, testing, and documentation, ensuring a seamless and thorough validation process.
- Regulatory Compliance: PharmEng Technology stays abreast of the latest regulatory changes and updates, ensuring that our clients’ systems meet current standards. Our proactive approach helps clients avoid potential compliance issues and ensures that systems are always audit-ready.
- Focus on Data Integrity: A core aspect of CSV is ensuring data integrity, and PharmEng Technology places a strong emphasis on this. Our validation processes are designed to prevent data corruption, errors, and unauthorized access, safeguarding the reliability and trustworthiness of critical data.
- Continuous Improvement: Understanding that validation is an ongoing process, PharmEng Technology offers continuous support and maintenance services. They assist clients with system updates, re-validation, and ongoing compliance checks, helping to maintain system integrity throughout its lifecycle.
- Training and Education: PharmEng Technology believes in empowering our clients through knowledge. They offer comprehensive training programs to ensure that client teams are well-versed in CSV principles and practices, fostering a culture of compliance and quality within the organization.
PharmEng Technology excels in providing Computerized System Validation services that not only meet regulatory requirements but also enhance system reliability, data integrity, and operational efficiency. Our dedication to quality and compliance makes them a trusted partner for organizations seeking to navigate the complexities of CSV with confidence.
About PharmEng Technology
PharmEng Technology is a global consulting firm specializing in pharmaceutical engineering, regulatory affairs, and compliance. With a commitment to quality and innovation, PharmEng Technology provides comprehensive solutions to meet the evolving needs of the pharmaceutical and biotechnology industries.
Contact Information
PharmEng Technology
Email: info.asia@pharmeng.com