
Complexity of Process Validation in Pharmaceutical Product Development
by Roseline Tio, Dhika Prameswari, and Izwan Firdaus (SEA CSV SME)
GMP guidelines emphasize process validation as a crucial part of product development, ensuring consistent quality and purity. Validated manufacturing processes are proven through data collection and evaluation, starting from the development phase and continuing through production. This includes process qualification and overall control for repeated batches.

Why is Validation Essential?
Pharmaceutical validation is indispensable for ensuring product consistency and safety during production. It encompasses regulating all raw materials and manufacturing techniques and thoroughly testing the finished product. This rigorous validation framework guarantees that each product not only meets but exceeds quality standards, safeguarding the health and well-being of consumers.
Phase of Process Validation
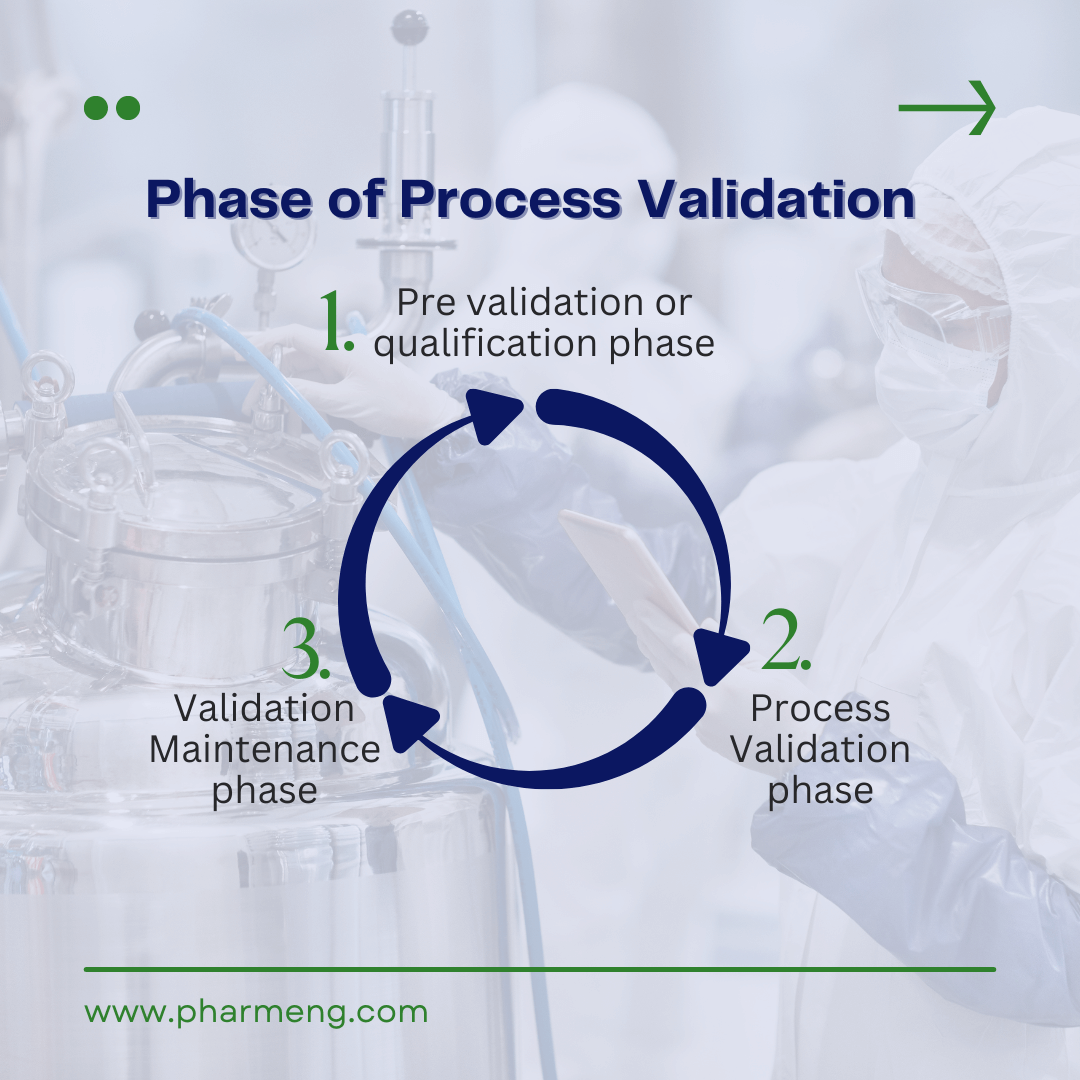
Phase 1: Pre-validation or qualification phase
The initial phase covers extensive groundwork, including product research, formulation development, scale-up studies, technology transfer, stability conditions, storage and handling protocols, equipment qualification, master production documents, operational qualification, and process capability assessments. This phase lays the foundation for robust manufacturing processes.
Phase 2: Process Validation phase
During this phase, the validity of critical process parameters is rigorously tested to ensure the production of satisfactory products, even under worst-case scenarios. This is achieved through comprehensive studies or trials that simulate various conditions, ensuring the process’s robustness and reliability to show:
- All systems, subsystems, or unit operations of a manufacturing process perform as intended.
- All critical parameters must operate within their assigned control limits.
- These studies and trials, which form the basis of process capability design and testing, are verifiable and certifiable through proper documentation.
Phase 3: Validation Maintenance phase
The final phase involves regularly reviewing process documents, including validation audit reports. This continuous monitoring ensures that no changes, deviations, failures, or modifications to the production process compromise product quality that should have resulted in requalification and revalidation. Consistent validation maintenance is critical to sustaining the high standards established during the initial phases.
At PharmEng Technology, our team of validation consultants brings a wealth of experience from various organizations. Our approach combines practical experience with professional knowledge, ensuring our work extends beyond mere documentation. Our engineers meticulously monitor each project stage, adhering to current regulatory and quality standards through rigorous data collection and analysis.

For more details on how we can support your validation objectives and contribute to your success in the pharmaceutical sector, please get in touch with us via email at info.asia@pharmeng.com. Discover how PharmEng Technology can be your partner in achieving excellence in pharmaceutical validation.
About PharmEng Technology
PharmEng Technology is a global consulting firm specializing in pharmaceutical engineering, regulatory affairs, and compliance. With a commitment to quality and innovation, PharmEng Technology provides comprehensive solutions to meet the evolving needs of the pharmaceutical and biotechnology industries.
Contact Information
PharmEng Technology
Email: info.asia@pharmeng.com





